Photoelectron radiation — physical meaning, laws and applications
The phenomenon of photoelectron emission (or external photoelectric effect) was discovered experimentally in 1887 by Heinrich Hertz during an open cavity experiment. When Hertz directed ultraviolet radiation at zinc sparks, at the same time the passage of an electric spark through them was noticeably easier.
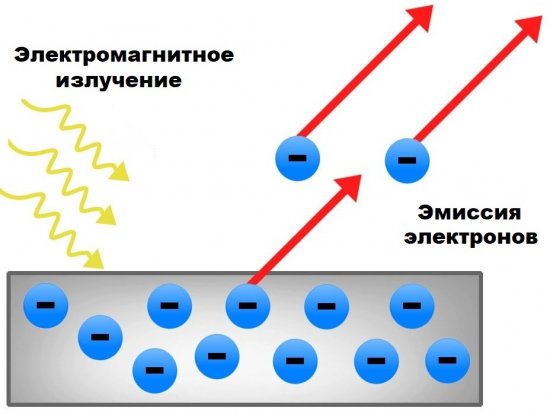
Thus, photoelectron radiation can be called the process of emission of electrons in a vacuum (or in another medium) from solid or liquid bodies under the influence of electromagnetic radiation falling on them. The most significant in practice is the photoelectron emission from solid bodies — in a vacuum.

1. Electromagnetic radiation with a constant spectral composition falling on the photocathode causes a saturated photocurrent I, the value of which is proportional to the irradiation of the cathode, that is, the number of photoelectrons knocked out (emitted) in 1 second is proportional to the intensity of the incident radiation F.
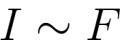
2.For each substance, in accordance with its chemical nature and with a certain state of its surface, which determine the work function Ф of electrons from a given substance, there is a long-wave (red) limit of photoelectron radiation, i.e. , the minimum frequency v0 below which the photoelectric effect is impossible.
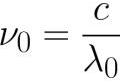
3. The maximum initial speed of the photoelectrons is determined by the frequency of the incident radiation and does not depend on its intensity. In other words, the maximum kinetic energy of photoelectrons increases linearly with increasing frequency of incident radiation and does not depend on the intensity of this radiation.
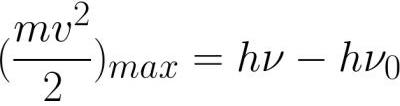
The laws of the external photoelectric effect would in principle be strictly satisfied only at absolute zero temperature, while in fact, at T > 0 K, photoelectron emission is also observed at wavelengths longer than the cut-off wavelength, albeit with a small number of emitting electrons. At an extremely high intensity of incident radiation (more than 1 W / cm 2 ), these laws are also violated, since the severity of multiphoton processes becomes obvious and significant.
Physically, the phenomenon of photoelectron emission is three consecutive processes.
First, the incident photon is absorbed by the substance, as a result of which an electron with energy higher than the average over the volume appears inside the substance. This electron moves to the surface of the body and along the way part of its energy is dissipated, because on the way such an electron interacts with other electrons and vibrations of the crystal lattice. Finally, the electron enters a vacuum or other medium outside the body, passing through a potential barrier at the boundary between these two mediums.
As is typical for metals, in the visible and ultraviolet parts of the spectrum, photons are absorbed by the conduction electrons. For semiconductors and dielectrics, electrons are excited from the valence band. In any case, a quantitative characteristic of photoelectron emission is the quantum yield — Y — the number of electrons emitted per incident photon.
The quantum yield depends on the properties of the substance, on the state of its surface, as well as on the energy of the incident photons.

In metals, the long-wavelength limit of photoelectron emission is determined by the work function of the electron from their surface. Most clean surface metals have a work function above 3 eV, while alkali metals have a work function of 2 to 3 eV.
For this reason, photoelectron emission from the surface of alkali and alkaline earth metals can be observed even when irradiated with photons in the visible region of the spectrum, not just UV. While in ordinary metals, photoelectron emission is only possible starting from UV frequencies.
This is used to reduce the work function of the metal: a film (monoatomic layer) of alkali and alkaline earth metals is deposited on an ordinary metal and thus the red limit of photoelectron emission is shifted to the region of longer waves.

The quantum yield Y characteristic of metals in the near-UV and visible regions is of the order of less than 0.001 electron/photon because the photoelectron leakage depth is small compared to the light absorption depth of the metal.The lion's share of photoelectrons dissipate their energy before even approaching the exit boundary of the metal, losing any chance of exit.
If the photon energy is close to the photoemission threshold, then most electrons will be excited at energies below the vacuum level and they will not contribute to the photoemission current. In addition, the reflection coefficient in the near UV and visible regions is too high for metals, so only a very small fraction of the radiation will be absorbed by the metal at all. In the far UV region these limits decrease and Y reaches 0.01 electron/photon at photon energies above 10 eV.
The figure shows the spectral dependence of the photoemission quantum yield for a pure copper surface:
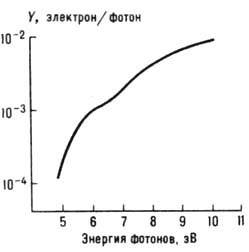
Contamination of the metal surface reduces the photocurrent and shifts the red limit to the longer wavelength region; at the same time, for the far UV region under these conditions, Y may increase.
Photoelectron radiation finds application in photoelectronic devices that convert electromagnetic signals of various ranges into electric currents and voltages. For example, an image in invisible infrared signals can be converted into a visible one using a device that works on the basis of the phenomenon of photoelectron emission. Photoelectron radiation also works in photocells, in various electronic-optical converters, in photomultipliers, photoresistors, photodiodes, in electron-beam tubes, etc.
See also:How the process of converting solar energy into electrical energy works