Electroluminescent emitters: device and principle of operation, types
Electroluminescence called luminescence, excited by the action of an electric field. This phenomenon occurs in semiconductors and crystalline phosphors — in such substances whose molecules or atoms are capable of going into an excited state when an electric current passes through them or under the action of an applied electric field.
In fact, electroluminescence results from the recombination of holes and electrons in a semiconductor, in which photons are emitted—the semiconductor's electrons thus giving up their energy. Before recombination begins, holes and electrons are separated. The separation is achieved either by high-energy electrons obtained by acceleration in a strong electric field (in crystalline phosphors of electroluminescent panels), or by activating the material to produce a pn junction (as in LEDs). In electroluminescent emitters, electroluminescence of an electroluminophore is used.
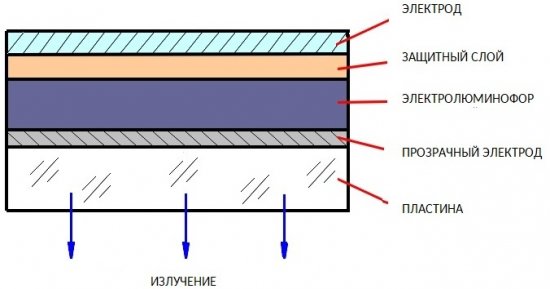
Powder Emitters were first developed in 1952.They are a multi-layered structure, at the base of which there is a plastic or glass substrate-plate.
The following is sequentially applied to the plate: a conductive transparent electrode made of metal oxides (SnO2, InO2, CdO), then a 25-100 μm layer of electroluminophore, then a protective dielectric layer (SiO, SiO2 or varnish), then an opaque metal electrode . Phosphorus is zinc sulfide or zinc selenide activated to brightness by impurities of manganese, copper, or other elements.
Zinc sulfide polycrystals (beads) are conjugated to each other by organic resins with a high dielectric constant. Therefore, to operate, the powder electroluminescent emitter requires an alternating voltage with a frequency of 400 to 1400 Hz with an excitation voltage of 90 to 140 volts.
Film electroluminescent emitters, unlike powder, contain between the electrodes a polycrystalline film of electroluminescent phosphor with a thickness of about 0.2 μm, which is obtained by thermal evaporation and vacuum deposition.
In such an electroluminophore, there is no dielectric, therefore film emitters work at a constant voltage, and their operating voltage level is less than that of powder ones - only from 20 to 30 volts. To increase the light and brightness, as well as to change the color, the film's phosphor is activated with rare earth fluoride materials.
The three-layer film emitter was created in 1974. It contains two insulating films (Y2O3 and Si3N4) with a high dielectric constant.
The characteristic parameters of electroluminescent emitters are: effective brightness, characteristic of brightness, frequency change in brightness, dependence of effective brightness on the frequency and spectrum of emitted light.
The effective brightness of the powder emitters is determined at a certain frequency and value of the alternating current supply voltage corresponding to the current density.
The brightness characteristic reflects the voltage dependence of the brightness; matrix screens with high contrast are built on the basis of emitters with a very non-linear characteristic.
Film emitters provide higher contrast and resolution than powder emitters. The multiple changes in luminance—in fact—the steepness of the luminance characteristic when the supply voltage is doubled; in powder it reaches 25, in film — 1000. The spectrum, in fact — the color, is determined by activators added to the phosphor.
Disadvantages of electroluminescent emitters include large variations in parameters. In addition, the brightness during their operation decreases up to 3 times in 4000 hours. But this applies to the first electroluminophores with large particles.
The latest modern electroluminophores have particle sizes of 12-18 nm, with them the brightness increases to 300 cd, and the decrease in brightness by 20% during the first 40 hours of operation is regulated by the power supply parameters (frequency and excitation voltage), and the operational life in this way reaches 12000 hours...
Different designs of opaque electrodes allow different alphabetic, symbolic and numerical forms of information display to be achieved using electroluminescent emitters to build on this special matrix screens.
Electroluminescent panels are available as thin films of inorganic or organic materials. The color of the glow of crystalline phosphors depends on the activating impurity.Basically, such a panel is a flat capacitor fed by a voltage of 60 to 600 volts obtained from a built-in voltage converter.
As electroluminescent materials are used: III-V InP, GaAs, GaN (in LEDs), zinc sulfide activated by silver or copper in the form of powder (gives a blue-green glow), and to obtain a yellow-orange glow, zinc uses se sulfide activated by manganese.
Electroluminescent Display (ELD) — a special type of display created by a layer of electroluminescent material consisting of specially processed phosphor or GaAs crystals between two layers of conductor (between a thin aluminum electrode and a transparent electrode). When an alternating voltage is applied to the wires, the electroluminescent material begins to glow.
Panels, displays, wires, etc. — widely used in consumer electronics and lighting electroluminescent illuminators… They serve in the backlight of LCD displays, scales of various devices, keyboards, and are also used for decorative design of landscapes and architectural structures.
Graphics from electroluminescent displays, synthesizing characters, characterized by high image quality, good contrast, high refresh rate and poor sensitivity to temperature. Due to these properties, they are used in the military, medical and other industries.