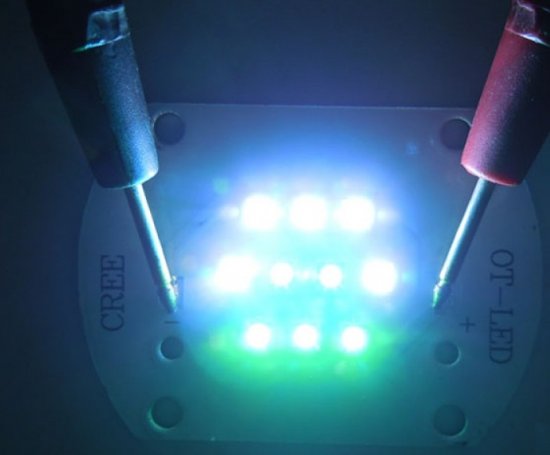
Luminescence — mechanism and application in light sources
Luminescence is the luminescence of a substance that occurs in the process of converting the energy absorbed by it into optical radiation. This glow is not caused directly by heating the substance.
The mechanism of the phenomenon is related to the fact that, under the influence of an internal or external source, atoms, molecules or crystals are excited in a substance, which then emits photons.
Depending on the duration of the luminescence thus obtained, which in turn depends on the lifetime of the excited state, a distinction is made between rapidly decaying and long-lasting luminescence. The first is called fluorescence, the second is phosphorescence.
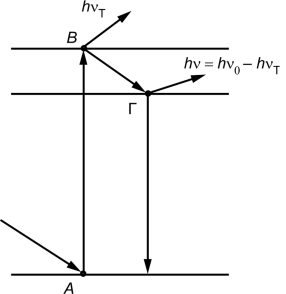
For a substance to glow, its spectra must be discrete, that is, the energy levels of the atoms must be separated from each other by forbidden energy bands. For this reason, solid and liquid metals that have a continuous energy spectrum do not luminesce at all.
In metals, the excitation energy is simply continuously converted to heat.And only in the short-wave range can metals experience X-ray fluorescence, that is, under the action of X-rays, they emit secondary X-rays.
Luminescence excitation mechanisms
There are different mechanisms for the excitation of luminescence, according to which there are several types of luminescence:
- Photoluminescence — excited by light in the visible and ultraviolet ranges.
-
Chemiluminescence — induced by a chemical reaction.
-
Cathodoluminescence — excited by cathode rays (fast electrons).
-
Sonoluminescence is excited in a liquid by an ultrasound wave.
-
Radioluminescence — excited by ionizing radiation.
-
Triboluminescence is excited by rubbing, crushing, or separating phosphors (electrical discharges between charged fragments), and in this case the discharge light excites photoluminescence.
-
Bioluminescence is the glow of living organisms, achieved by them independently or with the help of other participants in symbiosis.
-
Electroluminescence — excited by an electric current passed through a phosphor.
-
Candoluminescence is a luminous glow.
-
Thermoluminescence is excited by heating a substance.
The use of luminescence in light sources
Luminescent light sources are those whose glow is based on the phenomenon of luminescence. So all gas discharge lamps are fluorescent and mixed radiation sources. In photoluminescent lamps, the glow is created by a phosphor excited by the emission of an electric discharge.
White LEDs are usually based on blue InGaN crystal and yellow phosphor.The yellow phosphors used by most manufacturers are a modification of yttrium-aluminum garnet alloyed with trivalent cerium.
The luminescence spectrum of this phosphor has a characteristic maximum wavelength in the region of 545 nm. The long-wave portion of the spectrum dominates the short-wave portion. Modification of the phosphor with the addition of gallium and gadolinium makes it possible to shift the maximum of the spectrum to the cold region (gallium) or to the warm region (gadolinium).
Judging by the spectrum of the phosphor used in Cree LEDs, in addition to yttrium-aluminum garnet, a phosphor with a maximum emission shifted to the red region is added to the white LED phosphor.
In comparison with fluorescent lampsThe phosphor used in LEDs has a long service life, and the aging of the phosphor is mainly determined by temperature. The phosphor is usually applied directly to the LED crystal, which gets very hot. Other factors affecting phosphorus have a less pronounced effect on their service life.
The aging of the phosphor leads not only to a decrease in the brightness of the LED, but also to a change in the shade of the resulting light. With significant deterioration of the phosphor, the blue hue of the luminescence becomes clearly visible. This is due to the changing properties of the phosphor and the fact that the spectrum begins to dominate the internal emission of the LED chip. With the introduction of the technology of the isolated layer of phosphorus, the influence of temperature on the rate of its degradation decreases.
Other applications of luminescence
Photonics mainly uses converters and light sources based on electroluminescence and photoluminescence: LEDs, lamps, lasers, luminescent coatings, etc. — this is precisely the field in which luminescence is used very widely.
In addition, luminescence spectra help scientists in studying the composition and structure of substances. Luminescence methods make it possible to determine the size, concentration and spatial distribution of nanoparticles, as well as the lifetime of excited states of non-equilibrium charge carriers in semiconductor structures.
Continuing this thread:Electroluminescent emitters: device and principle of operation, types