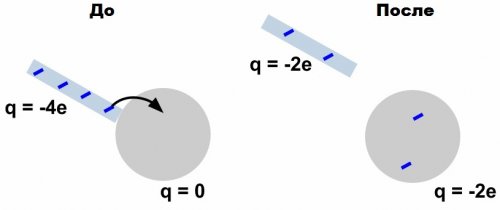
Law of Conservation of Electric Charge
Whatever happens in the world, there is a certain total electric charge in the universe, the size of which always remains unchanged. Even if the charge for some reason ceases to exist in one place, it will certainly end up in another place. This means that the charge cannot disappear forever.
This fact was established and investigated by Michael Faraday. He once erected a huge hollow metal ball in his laboratory, to the outer surface of which he connected an ultra-sensitive galvanometer. The size of the ball made it possible to place an entire laboratory inside it.
And so did Faraday. He began to bring into the ball the most varied electrical equipment at his disposal, and then began to experiment. Being in the ball, he began to rub glass rods with fur, start electrostatic machines, etc. But no matter how hard Faraday tried, the charge of the ball did not increase. In no way did the scientist manage to create a charge.
And we understand this because when you rub a glass rod with a fur, even though the rod gets a positive charge, the fur immediately gets a negative charge by the same amount, and the sum of the charge on the fur and the rod is zero.
A galvanometer outside the ball would certainly reflect the fact of a change in charge if an "extra" charge appeared in Faraday's laboratory, but nothing of the sort happened. Full charge is saved.
Another example. A neutron is initially an uncharged particle, but a neutron can decay into a proton and an electron. And although the neutron itself is neutral, that is, its charge is zero, the particles born as a result of its decay carry electrical charges of opposite sign and equal in number. The total charge of the universe has not changed at all, it remains constant.
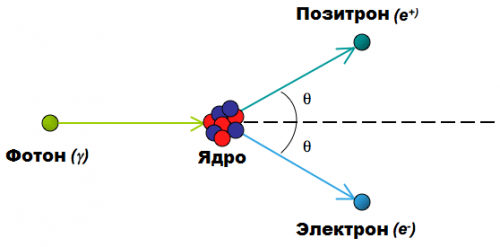
Another example is a positron and an electron. The positron is the antiparticle of the electron, it has the opposite charge of the electron and is essentially a mirror image of the electron. Once they meet, the electron and positron annihilate each other as a gamma-quantum (electromagnetic radiation) is born, but the total charge again remains unchanged. The reverse process is also true (see figure above).
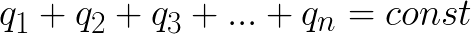
The law of conservation of electric charge is formulated as follows: the algebraic sum of charges of an electrically closed system is conserved. Or like this: with each interaction of the bodies, their total electric charge remains unchanged.
Electric charge changes in parts (quantized)
Electric charge has an unusual property—it always changes in parts. Consider a charged particle. Its charge can be, for example, one part of the charge or two parts of the charge, minus one or minus two parts.An elementary (minimum actually existing long-lived particles) negative charge has an electron.
The electron charge is 1.602 176 6208 (98) x 10-19 Pendant. This amount of charge is the minimum part (a quantum of electric charge). The minute pieces of electric charge can move in varying amounts from one place in space to another, but the total charge is always and everywhere conserved, and in principle can be measured as the number of these minute pieces.
Electric charges are sources of electric and magnetic fields
It is worth noting that electric charges are sources of electric and magnetic fields… Therefore, the electrical approach makes it possible to determine the amount of charge on one or another of its carriers. Also, charge is a measure of the interaction of a charged body with an electric field. As a result, electricity can be argued to be a phenomenon associated with charges at rest (static electricity, electric field) or moving (current, magnetic field).