Polar and non-polar dielectrics
According to the views of classical physics, dielectrics are fundamentally different from conductors, because under normal conditions there are no free electric charges in them. The total charge of the particles forming dielectric molecules is zero. However, this does not mean at all that the molecules of these substances are not capable of exhibiting electrical properties.
All known linear dielectrics can be divided into two large groups: polar dielectrics and nonpolar dielectrics. This division is introduced due to the differences in the polarization mechanisms of the molecules of each type of dielectric. In fact, the polarization mechanism turns out to be an extremely important aspect in the study of both the physical and chemical properties of dielectrics, and in the study of their electrical properties.
Nonpolar dielectrics
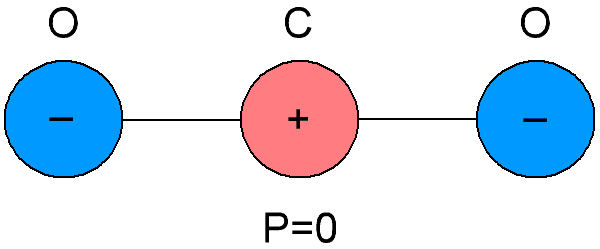
Non-polar dielectrics are also called neutral dielectrics, because the molecules of which these dielectrics are composed differ in the coincidence of the centers of gravity of the negative and positive charges inside them.As a result, it turns out that the molecules of non-polar dielectrics do not have their own electric moment, it is equal to zero. And in the absence of an external electric field, the positive and negative charges of the molecules of such substances are arranged symmetrically.
If an external electric field is applied to a non-polar dielectric, then the positive and negative charge in the molecules will be displaced from their original equilibrium position, the molecules will become dipoles whose electric moments will now be proportional to the strength of the electric field applied to them, and will be directed parallel to the field.
Examples of non-polar dielectrics that are successfully used today as electrical insulating materials are the following: polyethylene, polystyrene, hydrocarbons, petroleum insulating oils, etc. Also, bright representatives of non-polar molecules are, for example, nitrogen, carbon dioxide, methane, etc. Mr.
Nonpolar dielectrics, because of their low dielectric loss tangent values, are widely used as high-frequency dielectrics in capacitors such as the K78-2.
Polar dielectrics
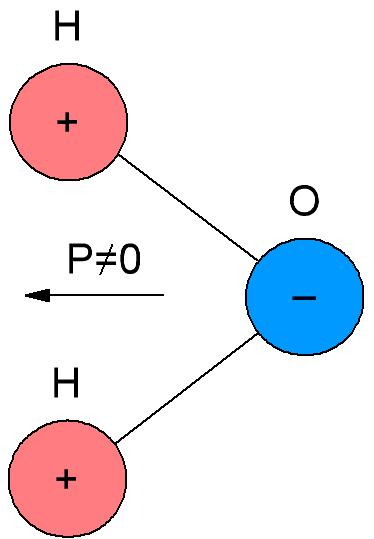
In polar dielectrics, which are also called dipole dielectrics, the molecules have their own electric moment, that is, their molecules are polar. The reason is that the molecules of polar dielectrics have an asymmetric structure, so the centers of mass of negative and positive charges in the molecules of such dielectrics do not coincide.
If in a non-polar polymer some of the hydrogen atoms are replaced by atoms of other elements or by non-hydrocarbon radicals, then we will get only a polar (dipole) dielectric, since the symmetry will be broken as a result of such a replacement. Determining the polarity of a substance by its chemical formula, the researcher must, of course, have an idea of the spatial structure of its molecules.
When there is no external electric field, the axes of the molecular dipoles are oriented arbitrarily due to thermal motion, so that on the surface of the dielectric and in every element of its volume the electric charge is on average zero. However, when a dielectric is introduced into an external field, a partial orientation of molecular dipoles occurs. As a result, uncompensated macroscopically connected charges appear on the surface of the dielectric, creating a field directed to the external field.
Examples of polar dielectrics include the following: chlorinated hydrocarbons, epoxy and phenol formaldehyde resins, silicon silicon compounds, etc. Water and alcohol molecules, for example, are also notable examples of polar molecules. Polar dielectrics are widely used in various fields of technology, such as piezoelectric and ferroelectric, optics, nonlinear optics, electronics, acoustics, etc.

