Organic semiconductors
The use of organic semiconductors extends to many areas of electronics: they are applicable as light-sensitive materials for recording information, they are used in the manufacture of sensors. Devices made on the basis of organic semiconductors are resistant to radiation, which is why they can be used even in open space and in nuclear technologies.
Organic semiconductors include solid organic compounds that initially have or acquire under the influence of external factors hole or electronic conductivity, as well as a positive temperature coefficient of electrical conductivity.
Semiconductors of this structure are characterized by the presence of conjugated aromatic rings in the molecules. Due to the excitation of p-electrons delocalized along the conjugated bonds, current carriers are formed in organic semiconductors. Moreover, the activation energy of these electrons decreases with an increase in the number of conjugations in the structure, and in polymers it can reach the level of thermal energy.
The property of conductivity in organic semiconductors is based on the movement of charge carriers both within the molecule and between molecules. As a result, high molecular weight semiconductors have a resistance of 10 ^ 5 to 10 ^ 9 Ohm * cm at room temperature, and low molecular weight semiconductors - from 10 ^ 10 to 10 ^ 16 Ohm * cm. And unlike ordinary semiconductors, there is no pronounced impurity conduction at low temperatures.
In reality, organic semiconductors exist in the form of amorphous or polycrystalline powders, films, and single crystals. Semiconductors in this context can be molecular crystals and complexes, organometallic complexes, as well as pigments and polymer semiconductors.
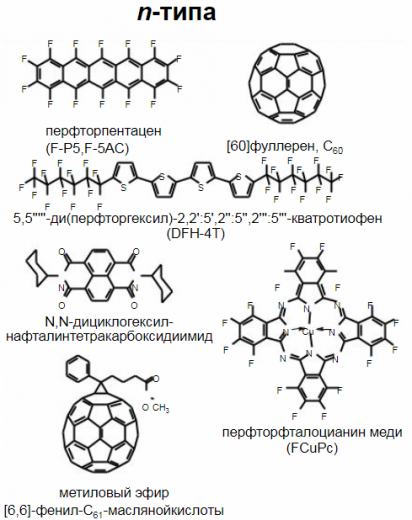
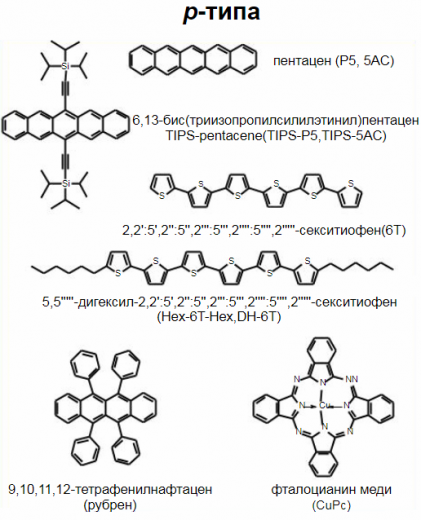
Molecular crystals are polycyclic aromatic low molecular weight crystalline compounds containing aromatic rings with a system of conjugated double bonds. Molecular crystals include phenanthrene, anthracene C14H10, naphthalene C10H8, phthalocyanines, etc.
Organometallic complexes include low molecular weight substances with a metal atom in the center of the molecule. These materials are polymerizable. A prominent representative of the organometallic complex is copper phthalocyanine.
Molecular complexes are low molecular weight polycyclic compounds with intermolecular electronic interactions. By their structure, molecular complexes are homogeneous and layered (with p-type and n-type layers). Halogenaromatic complexes are characterized by a homogeneous structure and layers, for example, anthracene compounds with alkali metals.
Polymeric semiconductors are compounds that have extended conjugation chains in macromolecules and have a complex structure.The longer the conjugation chain, the higher the specific electrical conductivity of the substance.
Pigments have semiconductor properties: eosin, indigo, radoflavin, trypaflavin, pinacyanol, rhadamine, etc. And from natural pigments - carotene, chlorophyll, etc.