Electric current in semiconductors
Between the conductors and dielectrics, in terms of resistance, are located semiconductors… Silicon, germanium, tellurium, etc. — many elements of the periodic table and their compounds belong to semiconductors. Many inorganic substances are semiconductors. Silicon is wider than others in nature; the earth's crust consists of 30% of it.
The main striking difference between semiconductors and metals lies in the negative temperature coefficient of resistance: the higher the temperature of the semiconductor, the lower its electrical resistance. For metals, it is the opposite: the higher the temperature, the greater the resistance. If a semiconductor is cooled to absolute zero, it becomes dielectric.

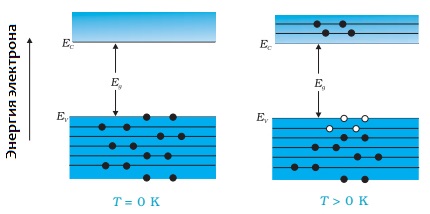
This dependence of semiconductor conductivity on temperature shows that the concentration free taxi drivers in semiconductors is not constant and increases with temperature.The mechanism of passage of an electric current through a semiconductor cannot be reduced to the model of a gas of free electrons, as in metals. To understand this mechanism, we can look at it for example on a germanium crystal.
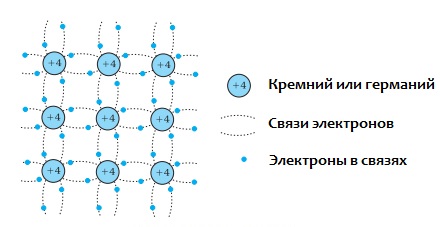
In the normal state, germanium atoms contain four valence electrons in their outer shell—four electrons that are loosely bound to the nucleus. Furthermore, each atom in the germanium crystal lattice is surrounded by four neighboring atoms. And the bond here is covalent, which means it is formed by pairs of valence electrons.
It turns out that each of the valence electrons belongs to two atoms at the same time, and the bonds of the valence electrons inside germanium with its atoms are stronger than in metals. That is why, at room temperature, semiconductors conduct current several orders of magnitude worse than metals. And at absolute zero, all of germanium's valence electrons will be occupied in bonds and there will be no free electrons to provide the current.
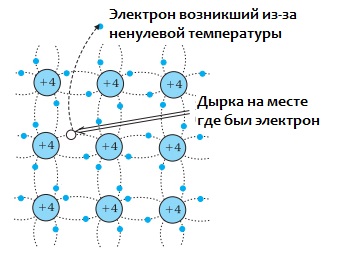
As the temperature increases, some of the valence electrons gain energy that becomes sufficient to break covalent bonds. This is how free conduction electrons arise. A type of vacancy is formed in disconnection zones— holes without electrons.
This hole can easily be occupied by a valence electron from a neighboring pair, then the hole will move into place at the neighboring atom. At a certain temperature, a certain number of so-called electron-hole pairs are formed in the crystal.
At the same time, the process of electron-hole recombination takes place — a hole meeting a free electron restores the covalent bond between atoms in a germanium crystal. Such pairs, consisting of an electron and a hole, can arise in a semiconductor not only due to temperature action, but also when the semiconductor is illuminated, that is, due to the energy incident on it electromagnetic radiation.
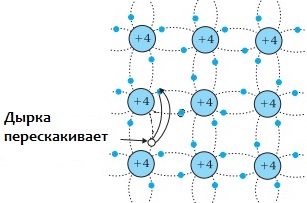
If no external electric field is applied to the semiconductor, then the free electrons and holes engage in chaotic thermal motion. But when a semiconductor is placed in an external electric field, the electrons and holes begin to move in an ordered manner. That's how it's born semiconductor current.
It consists of electron current and hole current. In a semiconductor, the concentration of holes and conduction electrons are equal. And only in pure semiconductors does it do so electron hole conduction mechanism… This is the intrinsic electrical conductivity of the semiconductor.
Impurity conduction (electron and hole)
If there are impurities in the semiconductor, then its electrical conductivity changes significantly compared to the pure semiconductor. Adding an impurity in the form of phosphorus to a silicon crystal, in an amount of 0.001 atomic percent, will increase the conductivity by more than 100,000 times! Such a significant effect of impurities on conductivity is understandable.
The main condition for the growth of impurity conductivity is the difference between the valence of the impurity and the valence of the parent element. Such impurity conduction is called impurity conduction and can be an electron and a hole.
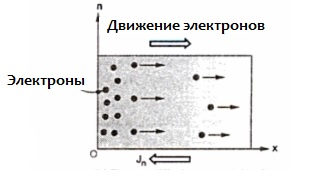
A germanium crystal begins to have electronic conductivity if pentavalent atoms, say, arsenic, are introduced into it, while the valence of the atoms of germanium itself is four. When the pentavalent arsenic atom is in place of the germanium crystal lattice, the four outer electrons of the arsenic atom are involved in covalent bonds with four neighboring germanium atoms. The fifth electron of the arsenic atom becomes free, it easily leaves its atom.
And the atom left by the electron turns into a positive ion in the place of the crystal lattice of the semiconductor. This is the so-called donor impurity when the valence of the impurity is greater than the valence of the main atoms. Many free electrons appear here, which is why, with the introduction of an impurity, the electrical resistance of the semiconductor drops thousands and millions of times. A semiconductor with a large amount of added impurities approaches metals in conductivity.
Although electrons and holes are responsible for the intrinsic conductivity in an arsenic-doped germanium crystal, the electrons that have left the arsenic atoms are the main free charge carriers. In such a situation, the concentration of free electrons greatly exceeds the concentration of holes, and this type of conductivity is called the electronic conductivity of the semiconductor, and the semiconductor itself is called an n-type semiconductor.
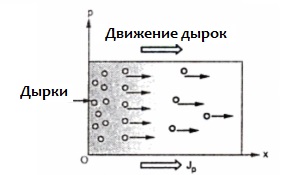
If, instead of pentavalent arsenic, trivalent indium is added to the germanium crystal, it will form covalent bonds with only three germanium atoms. The fourth germanium atom will remain unbonded to the indium atom. But a covalent electron can be captured by neighboring germanium atoms.The indium will then be a negative ion, and the neighboring germanium atom will occupy a vacancy where the covalent bond existed.
Such an impurity, when an impurity atom captures electrons, is called an acceptor impurity. When an acceptor impurity is introduced, many covalent bonds are broken in the crystal and many holes are formed into which electrons can jump from covalent bonds. In the absence of an electric current, the holes move randomly over the crystal.
An acceptor leads to a sharp increase in the conductivity of the semiconductor due to the creation of an abundance of holes, and the concentration of these holes significantly exceeds the concentration of electrons of the intrinsic electrical conductivity of the semiconductor. This is hole conduction and the semiconductor is called a p-type semiconductor. The main charge carriers in it are holes.
