Electric current in electrolytes
Electric current in electrolytes is always related to the transfer of matter. In metals and semiconductors, for example, matter when current passes through them is not transferred, because in these media electrons and holes are current carriers, but in electrolytes they are transferred. This is because in electrolytes, the positively and negatively charged ions of the substance act as carriers of free charges, not electrons or holes at all.
Molten compounds of many metals, as well as some solids, belong to electrolytes. But the main representatives of this type of conductors, which are widely used in technology, are aqueous solutions of inorganic acids, bases and salts.
The substance, when an electric current passes through the electrolyte medium, is released on the electrodes. This phenomenon is called electrolysis… When an electric current passes through the electrolyte, the positively and negatively charged ions of the substance move simultaneously in opposite directions.
Negatively charged ions (anions) rush to the positive electrode of the current source (anode), and positively charged ions (cations) to its negative pole (cathode).
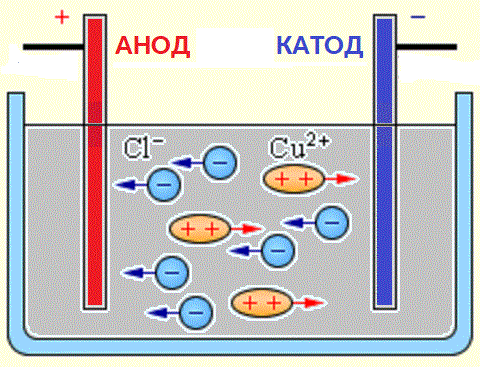
Sources of ions in aqueous solutions of acids, bases and salts are neutral molecules, some of which split under the action of an applied electrical force. This phenomenon of splitting neutral molecules is called electrolytic dissociation. For example, copper chloride CuCl2 decomposes on dissociation in aqueous solution into chloride ions (negatively charged) and copper (positively charged).
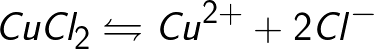
When the electrodes are connected to a current source, the electric field begins to act on ions in a solution or melt, as chlorine anions move to the anode (positive electrode) and copper cations to the cathode (negative electrode).
On reaching the negative electrode, the positively charged copper ions are neutralized by the excess electrons at the cathode and become neutral atoms that are deposited on the cathode. On reaching the positive electrode, the negatively charged chlorine ions donate one electron each during the interaction with the positive charge on the anode. In this case, the formed neutral chlorine atoms combine in pairs to form Cl2 molecules, and chlorine is released in the form of gas bubbles at the anode.
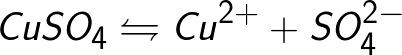
Often, the electrolysis process is accompanied by the interaction of dissociation products (this is called secondary reactions), when the decomposition products released on the electrodes interact with the solvent or directly with the electrode material. Take, for example, the electrolysis of an aqueous solution of copper sulfate (copper sulfate — CuSO4).In this example, the electrodes will be made of copper.
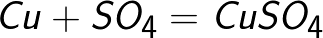
The copper sulfate molecule dissociates to form a positively charged copper ion Cu + and a negatively charged sulfate ion SO4-. Neutral copper atoms are deposited as a solid deposit on the cathode. In this way, chemically pure copper is obtained.
The sulfate ion donates two electrons to the positive electrode and becomes the neutral radical SO4, which immediately reacts with the copper anode (secondary anode reaction). The reaction product at the anode is copper sulfate, which goes into solution.
It turns out that when an electric current passes through an aqueous solution of copper sulfate, the copper anode simply gradually dissolves and copper precipitates on the cathode. In this case, the concentration of the aqueous solution of copper sulfate does not change.
In 1833, the English physicist Michael Faraday, in the course of experimental work, established the law of electrolysis, which is now named after him.
Faraday's law allows you to determine the amount of primary products that are released on the electrodes during electrolysis. The law states the following: "The mass m of the substance released on the electrode during electrolysis is directly proportional to the charge Q that has passed through the electrolyte."
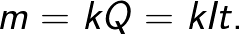
The proportionality factor k in this formula is called the electrochemical equivalent.
The mass of the substance that is released on the electrode during electrolysis is equal to the total mass of all ions that came to this electrode:
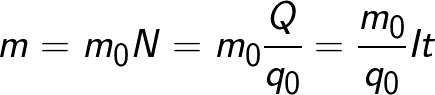
The formula contains the charge q0 and the mass m0 of an ion, as well as the charge Q that passed through the electrolyte. N is the number of ions that arrived at the electrode when the charge Q passed through the electrolyte.Therefore, the ratio of the mass of the ion m0 to its charge q0 is called the electrochemical equivalent of k.
Since the charge of an ion is numerically equal to the product of the valence of the substance and the elementary charge, the chemical equivalent can be represented in the following form:
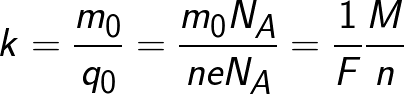
Where: Na is Avogadro's constant, M is the molar mass of the substance, F is Faraday's constant.
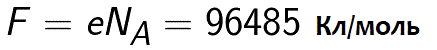
In fact, the Faraday constant can be defined as the amount of charge that must pass through the electrolyte to liberate one mole of monovalent substance on the electrode. Faraday's law of electrolysis then takes the form:
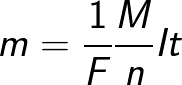
The phenomenon of electrolysis is widely used in modern production. For example, aluminum, copper, hydrogen, manganese dioxide and hydrogen peroxide are produced industrially by electrolysis. Many metals are extracted from ores and processed by electrolysis (electrorefining and electroextraction).
Also, thanks to electrolysis, chemical current sources… Electrolysis is used in wastewater treatment (electroextraction, electrocoagulation, electroflotation). Many substances (metals, hydrogen, chlorine, etc.) are obtained by electrolysis for electroplating and electroplating.
See also:Production of hydrogen by electrolysis of water — technology and equipment

