Faraday's laws of electrolysis
Faraday's laws of electrolysis are quantitative relationships based on Michael Faraday's electrochemical research, which he published in 1836.
These laws determine the relationship between the amount of substances released during electrolysis and the amount of electricity passed through the electrolyte. Faraday's laws are two. In the scientific literature and in textbooks, there are different formulations of these laws.
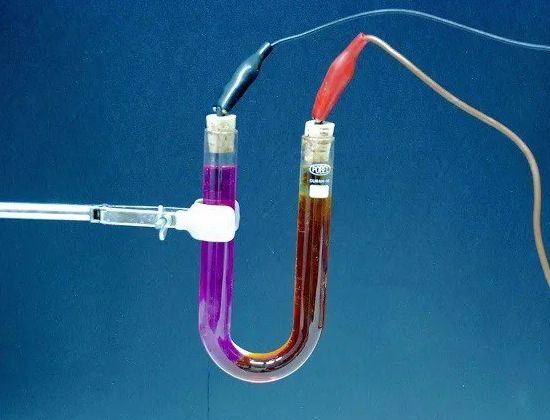
Electrolysis — release from the electrolyte of its constituent substances during the passage electricity… For example, when an electric current is passed through slightly acidified water, the water is decomposed into its constituent parts — gases (oxygen and hydrogen).
The amount of substance released from the electrolyte is proportional to the amount of electricity passing through the electrolyte, that is, the product of the strength of the current times the time during which this current flows. Therefore, the phenomenon of electrolysis can serve to measure the strength of the current and determine current units.
Electrolyte — a solution and generally a complex liquid that conducts an electric current.In batteries, the electrolyte is a solution of sulfuric acid (in lead) or a solution of caustic potash or caustic soda (in iron-nickel). In galvanic cells, solutions of any chemical compounds (ammonia, copper sulfate, etc.) also serve as an electrolyte.
Michael Faraday (1791 - 1867)
Michael Faraday (1791 — 1867) — English physicist, founder of the modern doctrine of electromagnetic phenomena. He began his working life as an apprentice in a bookbinding workshop. He received only an elementary education, but independently studied science and worked as a laboratory assistant for the chemist Devi, he became a great scientist, one of the greatest experimental physicists.
Farraday opened up phenomenon of electromagnetic induction, the laws of electrolysis, developed the doctrine of electric and magnetic fields and laid foundations of modern electromagnetic field concepts… He was the first scientist to have the idea of the vibrational, wave nature of electromagnetic phenomena.
Faraday's first law of electrolysis
The mass of a substance that will precipitate on an electrode during electrolysis is directly proportional to the amount of electricity transferred to that electrode (passed through the electrolyte). The amount of electricity refers to the amount of electrical charge, usually measured in pendants.
Faraday's second law of electrolysis
For a given amount of electricity (electrical charge), the mass of a chemical element that will be deposited on an electrode during electrolysis is directly proportional to the equivalent mass of that element. The equivalent mass of a substance is its molar mass divided by a whole number, depending on the chemical reaction in which the substance is involved.
Or
The same amount of electricity leads to the release of equivalent masses of different substances on the electrodes during electrolysis. To liberate one mole of the equivalent of any substance, it is necessary to spend the same amount of electricity, namely 96485 C. This electrochemical constant is called Faraday number.
Faraday's laws in mathematical form
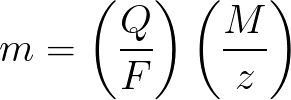
-
m is the mass of the substance deposited on the electrode;
-
Q is the value of the total electric charge in the pendants, passed during electrolysis;
-
F = 96485.33 (83) C / mol — Faraday's number;
-
M is the molar mass of the element in g/mol;
-
z — valence number of ions of a substance (electrons per ion);
-
M / z — equivalent mass of the substance applied to the electrode.
Applied to Faraday's first law of electrolysis, M, F, and z are constants, so the more Q, the more m will be.
In terms of Faraday's second law of electrolysis, Q, F and z are constants, so the more M / z, the more m will be.
For direct current we have
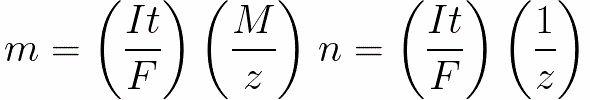
-
n is the number of moles (amount of substance) released on the electrode: n = m / M.
-
t is the time of passage of direct current through the electrolyte. For alternating current, the total charge is summed over time.
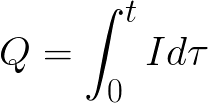
-
t is the total electrolysis time.
An example of applying Faraday's laws
It is necessary to write the equation of the electrochemical processes at the cathode and anode during the electrolysis of an aqueous solution of sodium sulfate with an inert anode. The solution to the problem will be the following. In solution, sodium sulfate will dissociate according to the following scheme:
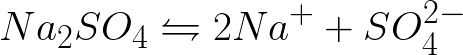
The standard electrode potential in this system is as follows:
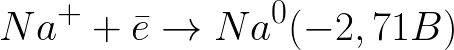
This is a much more negative potential level than for a hydrogen electrode in a neutral medium (-0.41 V). Therefore, on the negative electrode (cathode), the electrochemical dissociation of water will begin with the release of hydrogen and hydroxide ion according to the following scheme:
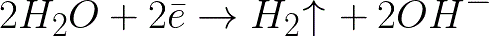
And the positively charged sodium ions approaching the negatively charged cathode will accumulate near the cathode, in the adjacent part of the solution.
Electrochemical oxidation of water will occur on the positive electrode (anode), which will lead to the release of oxygen, according to the following scheme:
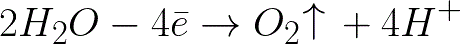
In this system, the standard electrode potential is +1.23 V, which is well below the standard electrode potential found in the following system:
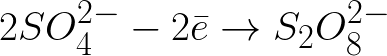
Negatively charged sulfate ions moving toward the positively charged anode will accumulate in the space near the anode.

