Magnetism of dielectrics and semiconductors
Unlike metals, dielectrics and semiconductors do not typically have itinerant electrons. Therefore, magnetic moments in these substances they are localized together with electrons in ionic states. This is the main difference. magnetism of metals, described by the band theory, by the magnetism of dielectrics and semiconductors.
According to band theory, dielectrics are crystals containing an even number electrons… This means that dielectrics can only expose diamagnetic properties, which, however, does not explain some of the properties of many substances of this type.
In fact, paramagnetism of localized electrons, as well as ferro- and antiferromagnetism (one of the magnetic states of a substance, characterized by the fact that the magnetic moments of neighboring particles of the substance are oriented towards each other, and therefore the magnetization of the body as a whole is very small) of dielectrics is the result of the Coulomb mutual repulsion of electrons (the Coulomb interaction energy of electrons Uc in real atoms ranges from 1 to 10 or more electron volts).
Suppose that an additional electron appeared in an isolated atom, which caused its energy to increase by the value e. This means that the next electron is in the energy level Uc + e. Inside the crystal, the energy levels of these two electrons split into bands, and as long as the band gap exists, the crystal is either a semiconductor or a dielectric.
Together, the two zones usually contain an even number of electrons, but a situation can arise where only the lower zone is filled and the number of electrons in it is odd.
Such a dielectric is called Mott-Hubbard dielectric… If the overlap integrals are small, then the dielectric will exhibit paramagnetism, otherwise there will be pronounced antiferromagnetism.
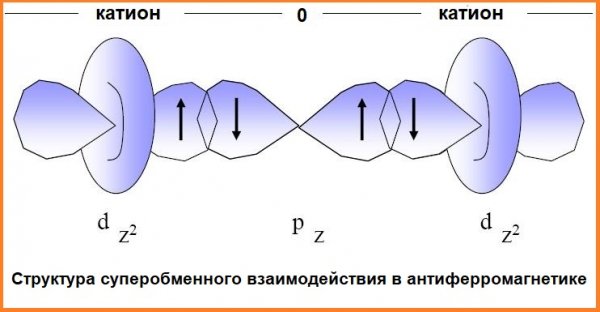
Dielectrics such as CrBr3 or EuO exhibit ferromagnetism based on superexchange interaction. The majority of ferromagnetic dielectrics consist of magnetic 3d-ions separated by non-magnetic ions.
In a situation where the distance for direct interaction of 3d-orbitals with each other is large, exchange interaction is still possible - by overlapping the wave functions of 3d-orbitals of magnetic ions and p-orbitals of non-magnetic anions.
Orbitals of two types "mix", their electrons become common to several ions - this is the superexchange interaction. Whether such a dielectric is ferromagnetic or antiferromagnetic is determined by the type of d-orbitals, the number of their electrons, and also by the angle at which a pair of magnetic ions is seen from where the nonmagnetic ion is located.
An antisymmetric exchange interaction (called the Dzialoszinski-Moria interaction) between two cells with spin vectors S1 and S2 has nonzero energy only if the cells in question are not magnetically equivalent.
An interaction of this type is observed in some antiferromagnets in the form of weak spontaneous magnetization (in the form of weak ferromagnetism), that is, the magnetization is a thousandth compared with magnetization of conventional ferromagnets… Examples of such substances: hematite, manganese carbonate, cobalt carbonate.