Graphite and its application in electrical engineering
The name «graphite» comes from the Greek word «grapho» - to write. This mineral is one of the modifications of carbon with a characteristic layered structure. Historical evidence of the use of graphite in antiquity as a colorant has been preserved - this is a clay vessel dating from the 40th century BC, painted with this mineral.
The modern name graphite was obtained in 1789 by the German geologist and teacher Abraham Gottlob Werner, who, among other things, studied sedimentary rock layers and also developed scales for determining minerals by external signs.
In nature, graphite is formed at a shallow depth, due to the metamorphism of rocks containing organic remains. In terms of physical and chemical properties, graphite is a crystalline refractory substance, slightly greasy to the touch, black or gray in color, with a characteristic metallic luster.
Compared to diamond, graphite is very soft due to the layered structure of the atomic lattice.Carbon atoms are found in graphite layer by layer, and the distance between the layers is greater than between the atoms in a single layer, and the electrons that connect the layers to each other form a continuous electron cloud - therefore graphite is a conductor of current and has a characteristic metallic shine.
With a density of 2.08 to 2.23 g/cm3, its electrical resistance at room temperature is 765 times that of copper.
Unlike diamond, graphite conducts electricity and heat well. The softness of graphite (mixed with kaolin) is applied in pencils. If you look at graphite under a microscope, it is easy to see the flakes, they remain on the paper, forming a mark when we use a pencil.
The physical and chemical characteristics of graphite opened its wide use in various electrical engineering. Due to its chemical resistance to aggressive aqueous solutions, fire-resistant properties and high electrical conductivity, electrodes and heating elements for various purposes are made of graphite. For example, in obtaining active metals by electrolysis, the electrodes are made of graphite.
When aluminum is obtained, the graphite itself leaves the reaction zone of the electrolyzer in the composition of carbon dioxide, so there is no need to apply other complicated measures for its disposal.
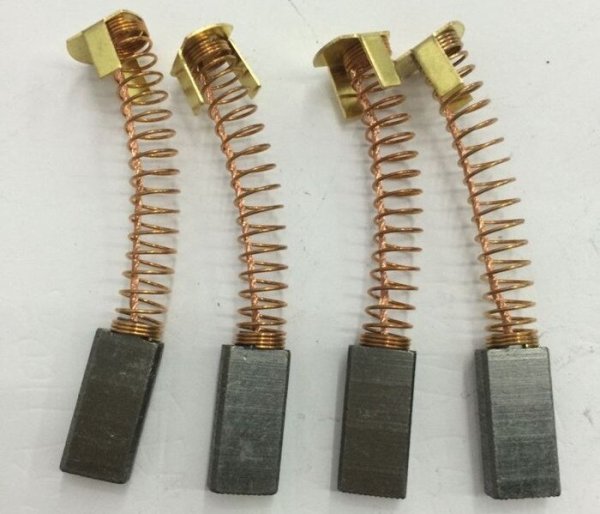
High resistance conductive adhesives contain only graphite as the conductive component. Well, everyone, of course, knows that it is from graphite that various contact brushes and current collectors of electrical equipment are made (collector motors of electric vehicles and cranes, contacts of current rheostats, etc.), where movable and in the same sometimes a reliable electrical outlet is needed...
But if we said that graphite is so soft, how are brushes made of collector assemblies that constantly rub against the contact plates and rings? After all, very often graphite brushes can be found in household appliances: in a mixer, electric shaver, coffee grinder, electric drill, grinder, etc. What is the secret here? Why don't brushes wear out instantly like a pencil?
But that's the point brushes for electrical engineering They are made not from pure graphite, but from graphite with the addition of a binder and even undergo special processing. The technology for the production of brushes is quite complex, it includes pressing and firing processes, which makes the brushes more durable and resistant to wear. .
So, at the last stage of production, the electrograft brushes are saturated with carbon in a furnace at a temperature of 2500 degrees! Metal graphite brushes contain metal powders and soot.
There are hard, medium and soft electrographic brushes. Soft brushes:
-
EG-4 and EG-71; EG -14 — medium, universal;
-
EG-8 and EG-74 are hard, contain abrasive powder.
Hard brushes are used in conditions of high temperatures and difficult commutation, so the abrasive included in the brush gives the brush an additional cleaning function, when the brush not only transfers current to the collector, but also immediately cleans it of carbon deposits.
Continuation of the topic:
What is the difference between graphene and graphite?