Resistance of alloys
There are many metals and many more alloys of several metals.
The earliest artificial alloys from human metallurgical experiments were created (based on archaeological remains) from about 3000 to 2500 BCE.
It is primarily bronze because the metals of which it is composed (copper and tin) are present (in abundance) in their native state and do not require extraction from the ore.
Gold and silver are metals that are abundant in nature and for this reason they are known from the 5th millennium BC, so they are also very often mixed, in particular to change the color or hardness of gold .
In theory, there are an infinite number of alloys. The basic process is simple: simply heat two or more metals until they reach the appropriate melting point, then mix them according to the correct dosages and start cooling them.
Thus, it is enough to even slightly change the dosage of the ingredients to create a new alloy that has unique properties.In addition, the production conditions of the new alloy are also crucial: it is enough, for example, to change the melting point, the firing conditions or even the cooling time.
The dependence of the resistance of alloys on their composition has a very different character. In some cases, the alloy is a collection of very small crystals of the two metals that make up the alloy. Each metal crystallizes independently of each other, after which their crystals are uniformly and rather randomly mixed in the alloy.
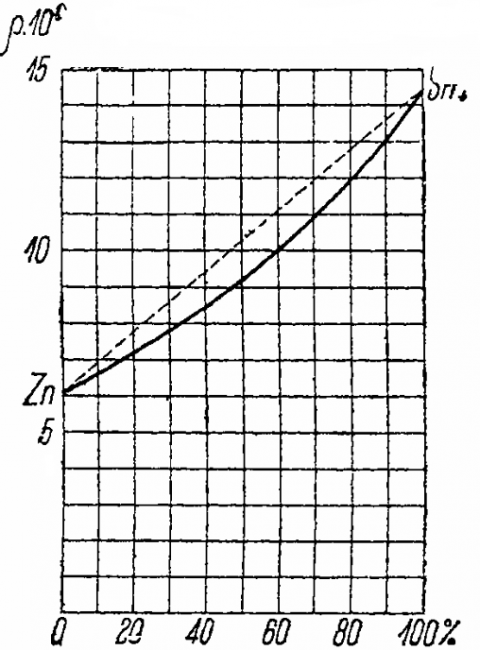
These are lead, tin, zinc and cadmium, which are mixed in any way. The resistance of such alloys at different concentrations lies between the extreme values of the resistance of pure metals, that is, it is always less than the larger of them and more than the smaller.
Metal Resistance Details: What determines the resistance of a conductor
Another useful article: Basic properties of metals and alloys
The figure below graphically shows the dependence of the resistivity of a zinc-tin alloy on the volume concentration of the two metals.
The abscissa shows the volumes of tin as a percentage of the alloy unit volume, i.e. abscissa 60 means that a unit volume of alloy contains 0.6 volume of tin and 0.4 volume of zinc. The ordinate shows the alloy resistivity values multiplied by 106.
Since pure metals temperature coefficients of resistance are quantities of the same order close to the coefficient of expansion of gases, it is obvious that the alloys of the considered group have coefficients of the same order.
In many other cases, the alloys of the two metals are a homogeneous mass composed of small crystals composed of atoms of the two metals.
Sometimes such mixed crystals can be formed from atoms of the two metals in any ratio, sometimes such formations are possible only in certain areas of concentration.
Outside these regions the alloys are similar to those of the first group just considered, except that they are a mixture of crystals of the pure metal and crystals of a mixed type composed of atoms of both types.
The resistivity of alloys of this type is usually greater than the resistivity of the two metals.

The figure below graphically shows the concentration dependence of the resistivity of an alloy of gold and silver forming mixed crystals at each concentration. The method of constructing the curve is the same as the curve in the previous figure.
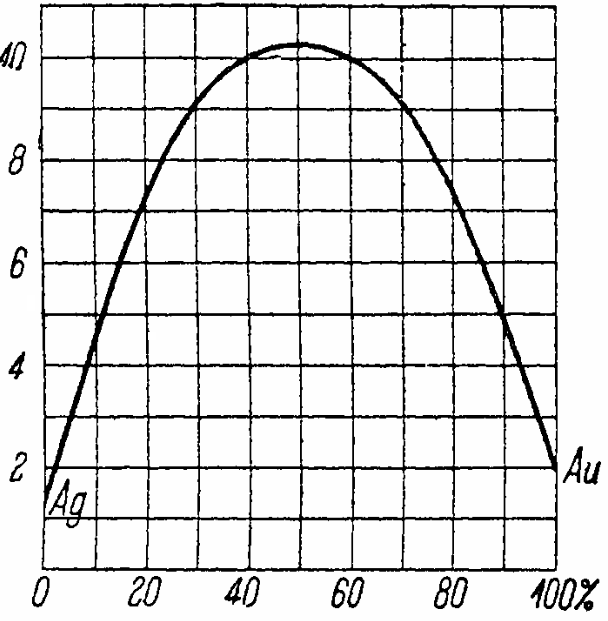
The resistance of pure silver on the graph is 1.5 * 10-6, pure gold 2.0 * 10-8... By alloying equal volumes of the two metals (50%), we get an alloy with a resistance of 10.4 * 10-6.
The temperature coefficients of resistance for alloys of this group are generally lower than for each of the metals that make up the alloy.
The figure below graphically shows the dependence of the temperature coefficient of an alloy of gold and silver on the concentration of gold.
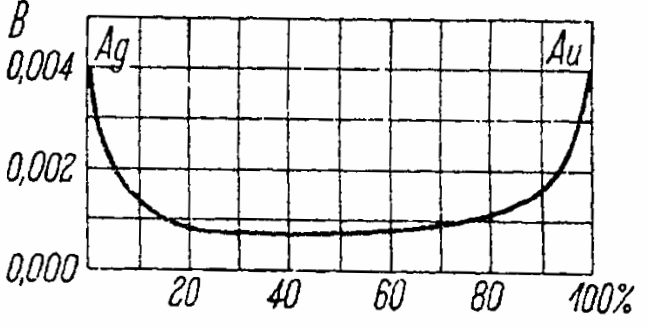
In the range of concentrations from 15% to 75%, the temperature coefficient of resistance does not exceed a quarter of the same coefficient of pure metals.
Some alloys of three metals are of technical importance.
The first of these alloys, manganin, when properly processed, has a temperature coefficient of zero, with the result that manganin wire is used to make precision resistance magazines.
An alloy of nickel, chromium, with additions of manganese, silicon, iron, aluminum (nichrome) is the most common material for the production of various heating elements.
More details about this type of alloys: Nichromes: varieties, composition, properties and characteristics
The remaining alloys (constantan, nickeline, nickel silver) are used for the manufacture of regulating rheostats because they have considerable resistance and are relatively little oxidized in air at those rather high temperatures which rheostat wires often have.
For more details on the ternary alloys most commonly used in the electrical industry, see here:High resistance materials, high resistance alloys
It is best to look up specific resistance values of various alloys in special reference books or determine experimentally, as they can vary widely.
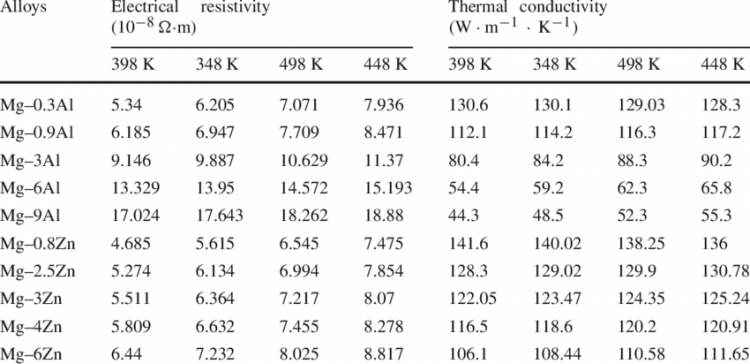
As an example, we give the values of electrical resistance and thermal conductivity of Mg-Al and Mg-Zn alloys:
In this work, the electrical resistivity and thermal conductivity of Mg — Al and Mg — Zn binary alloys are investigated in the temperature range from 298 K to 448 K and the relationship between the corresponding electrical conductivity and thermal conductivity of the alloys is analyzed.
See also: The most common conductive materials in electrical installations