Perfect electrical contact, influence of material properties, pressure and dimensions on contact resistance
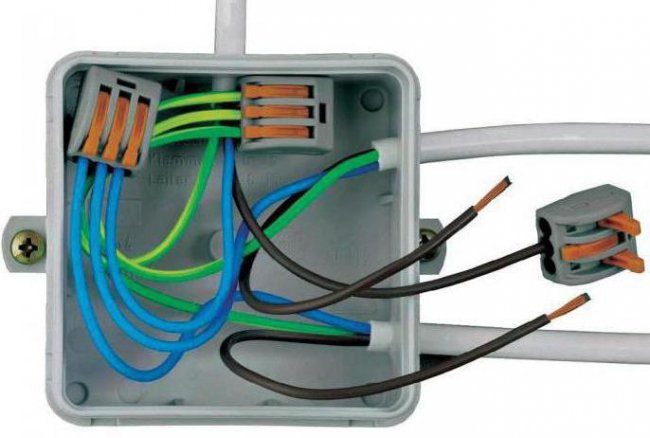
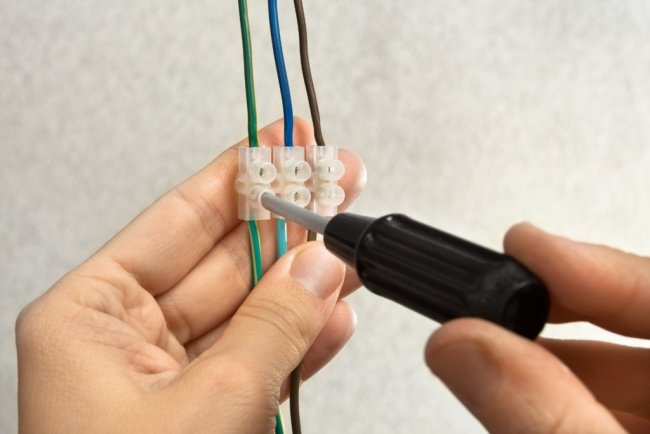
Fixed contacts are made in most cases by mechanical connection of wires, and the connection can be made either by direct connection of wires (for example, buses in electrical substations) or by intermediate devices - clamps and terminals.
Mechanically formed contacts are called tighteningand they can be assembled or disassembled without disturbing their individual parts. In addition to clamping contacts, there are fixed contacts obtained by soldering or welding the connected wires. We call such contacts all metal, as they have no physical boundary that delimits the two wires.
The reliability of the contacts in operation, the stability of the resistance, the absence of overheating and other disturbances determine the normal operation of the entire installation or line in which there are contacts.
The so-called ideal contact must meet two main requirements:
- the contact resistance must be equal to or lower than the resistance of the conductor in a section of the same length;
- contact heating with rated current must be equal to or lower than the heating of a wire of the corresponding cross-section.
In 1913, Harris developed four laws that govern electrical contacts (Harris F., Resistance of Electrical Contacts):
1. All other conditions being equal, the voltage drop in the contact increases in direct proportion to the current. In other words, the contact between two materials behaves as a resistance.
2. If the condition of the surfaces in the contact has no effect, the voltage drop across the contact varies inversely with the pressure.
3. The contact resistance between different materials depends on their specific resistance. Low resistivity materials also have low contact resistance.
4. The resistance of the contacts does not depend on the size of their area, but depends only on the total pressure in the contact.
The size of the contact surface is determined by the following factors: heat transfer conditions of the contacts and corrosion resistance, since a contact with a small surface can be destroyed by the penetration of corrosive agents from the atmosphere more easily than a contact with a large contact surface.
Therefore, when designing clamping contacts, it is necessary to know the norms of pressure, current density and size of the contact surface, which ensure compliance with the requirements for an ideal contact and which can be different depending on the material, surface treatment and contact design.
Contact resistance is affected by the following material properties:
1.Specific electrical resistance of the material.
The higher the contact resistance, the higher the specific resistance of the contact material.
2. The hardness or compressive strength of the material. The softer material deforms more easily and establishes contact points more quickly and therefore gives less electrical resistance at lower pressure. In this sense, it is useful to cover hard metals with softer ones: tin for copper and brass and tin or cadmium for iron.
3. Coefficients of thermal expansion It is also necessary to take into account, because due to their difference between the material of the contacts and, for example, bolts, increased stresses can occur, causing plastic deformation of the weaker part of the contact and its destruction with a decrease in temperature.
The amount of contact resistance is determined by the number and size of the point contacts and depends (to varying degrees) on the material of the contacts, the contact pressure, the treatment of the contact surfaces and the size of the contact surfaces.
At short circuits the temperature in the contacts can rise so much that due to the non-uniform coefficient of thermal expansion of the material of the bolts and the contact, stresses above the elastic limit of the material can occur.
This will cause loosening and loss of contact tightness. Therefore, when calculating, it is necessary to check for additional mechanical stresses in the contact caused by short-circuit currents.
Copper begins to oxidize in air at room temperature (20 — 30 °).The resulting oxide film, due to its small thickness, does not represent a particular obstacle to the formation of a contact, since it is destroyed when the contacts are compressed.
For example, contacts exposed to air for a month before assembly show only 10% more resistance than freshly made contacts. Strong oxidation of copper begins at temperatures above 70 °. The contacts, which were held for about 1 hour at 100 °, increased their resistance 50 times.
An increase in temperature significantly accelerates the oxidation and corrosion of contacts due to the fact that the diffusion of gases in the contact is accelerated and the reactivity of corrosive substances increases. The alternation of heating and cooling promotes the penetration of gases in contact.
It was also established that during prolonged heating of the contacts by current, a cyclical change in their temperature and resistance is observed. This phenomenon is explained by successive processes:
- oxidation of copper to CuO and increase in resistance and temperature;
- with lack of air, the transition from CuO to Cu2O and decreasing resistance and temperature (Cu2O conducts better than CuO);
- increased air access, new formation of CuO, increase in resistance and temperature, etc.
Due to the gradual thickening of the oxide layer, an increase in contact resistance is eventually observed.
The presence of sulfur dioxide, hydrogen sulfide, ammonia, chlorine and acid vapors in the atmosphere has a much stronger effect on contact with copper.
In the air, aluminum quickly becomes covered with a thin, highly resistant oxide film. The use of aluminum contacts without removing the oxide film gives high contact resistance.
Removal of the film at ordinary temperatures is possible only mechanically, and cleaning of the contact surface must be carried out under a layer of petroleum jelly to prevent air from reaching the cleaned surface. Aluminum contacts treated in this way give low contact resistance.
To improve contact and protect against corrosion, the contact surfaces are usually cleaned with petroleum jelly for aluminum and tin for copper.
When designing clamps for connecting aluminum wires, it is necessary to take into account the property of aluminum to "shrink" over time, as a result of which the contact weakens. Taking into account this property of aluminum wires, it is possible to use special terminals with a spring, due to which the necessary contact pressure is maintained in connection at all times.
Contact pressure is the most significant factor affecting contact resistance. In practice, the contact resistance depends mainly on the contact pressure and to a much lesser extent on the treatment or size of the contact surface.
An increase in contact pressure causes:
- reduction of contact resistance:
- loss reduction;
- tight bonding of the contact surfaces, which reduces the oxidation of the contacts and thus makes the connection more stable.
In practice, the normalized contact pressure is usually used, where contact resistance stability is achieved. Such optimum contact pressure values are different for different metals and different states of the contact surfaces.
An important role is played by the contact density over the entire surface, for which the specific pressure norms must be maintained regardless of the size of the contact surface.
The treatment of the contact surfaces must ensure the removal of foreign films and give maximum point contacts when the surfaces are in contact.
Covering the contact surfaces with a softer metal, such as tinning copper or iron contacts, makes it easier to achieve good contact at lower pressures.
For aluminum contacts, the best treatment is to sand the contact surface with sandpaper under petroleum jelly. Petroleum jelly is necessary because aluminum in air very quickly becomes covered with an oxide film, and petroleum jelly prevents air from reaching the protected contact surface.
A number of authors believe that the contact resistance depends only on the total pressure in the contact and does not depend on the size of the contact surface.
This can be imagined if, for example, with a decrease in the contact surface, the increase in contact resistance due to a decrease in the number of contact points is compensated by a decrease in resistance due to their flattening due to an increase in the specific contact pressure.
Such a mutual compensation of two oppositely directed processes can occur only in exceptional cases. Many experiments show that as the contact length decreases and at a constant total pressure, the contact resistance increases.
With the halved contact length, resistance stability is achieved at higher pressures.
The reduction of contact heating at a given current density is facilitated by the following properties of the contact material: low electrical resistance, high heat capacity and thermal conductivity, as well as a high ability to radiate heat on the outer surface of the contacts.
Corrosion of contacts made of different metals is much more intense than that of contacts made of the same metals. In this case, an electrochemical macrocouple is formed (metal A — wet film — metal B), which is a galvanic cell. Here, as in the case of microcorrosion, one of the electrodes will be destroyed, namely the part of the contact consisting of a less noble metal (anode).
In practice, there may be cases of connecting wires consisting of different metals, for example, copper with aluminum. Such a contact, without special protection, can corrode the less precious metal, i.e. aluminum. In fact, aluminum in contact with copper is highly corrosive, so direct bonding in contact between copper and aluminum is not allowed.