Batteries. Calculation examples
 Batteries are electrochemical current sources that, after discharge, can be charged using electric current drawn from a charger. When the charging current flows in the battery, electrolysis occurs, as a result of which chemical compounds are formed on the anode and cathode that were on the electrodes in the initial operating state of the battery.
Batteries are electrochemical current sources that, after discharge, can be charged using electric current drawn from a charger. When the charging current flows in the battery, electrolysis occurs, as a result of which chemical compounds are formed on the anode and cathode that were on the electrodes in the initial operating state of the battery.
Electrical energy, when charged in a battery, is converted into a chemical form of energy. When it is discharged, the chemical form of energy becomes electrical. It takes more energy to charge a battery than can be obtained by discharging it.
The voltage of each cell of a lead-acid battery after charging 2.7 V should not drop below 1.83 V when discharging.
The average voltage of a nickel-iron battery is 1.1 V.
The charging and discharging currents of the battery are limited and set by the manufacturer (approximately 1 A per 1 dm2 of the plate).
The amount of electricity that can be drawn from a charged battery is called the ampere-hour capacity of the battery.
Batteries are also characterized by energy and current efficiency.The energy return is equal to the ratio of the energy received during discharge to the energy spent charging the battery: ηen = Araz / Azar.
For a lead-acid battery ηen = 70% and for an iron-nickel battery ηen = 50%.
The current output is equal to the ratio of the amount of electricity received during discharge to the amount of electricity consumed during charging: ηt = Q times / Qchar.
Lead-acid batteries have ηt = 90% and iron-nickel batteries ηt = 70%.
Battery calculation
1. Why is the current return of the battery greater than the energy return?
ηen = Araz / Azar = (Up ∙ Ip ∙ tp) / (Uz ∙ Iz ∙ tz) = Up / Uz ∙ ηt.
The energy return is equal to the current return ηt multiplied by the ratio of the discharge voltage to the charge voltage. Since the ratio Uр / U3 <1, then ηen <ηt.
2. A lead-acid battery with a voltage of 4 V and a capacity of 14 Ah is shown in fig. 1. The connection of the plates is shown in fig. 2. Connecting the plates in parallel increases the battery capacity. Two sets of plates are connected in series to increase the voltage.
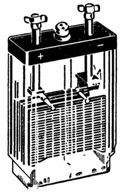
Rice. 1. Lead-acid battery
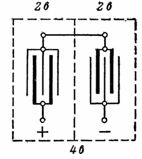
Rice. 2. Connecting the plates of a lead-acid battery for a voltage of 4 V
The battery is charged in 10 hours with a current of Ic = 1.5 A and discharged in 20 hours with a current of Ip = 0.7 A. What is the current efficiency?
Qp = Ip ∙ tp = 0.7 ∙ 20 = 14 A • h; Qz = Iz ∙ tz = 1.5 ∙ 10 = 15 A • h; ηt = Qp / Qz = 14/15 = 0.933 = 93%.
3. The battery is charged with a current of 0.7 A for 5 hours. How long will it discharge with a current of 0.3 A with a current output ηt = 0.9 (Fig. 3)?
Rice. 3. Figure and diagram for example 3
The amount of electricity used to charge the battery is: Qz = Iz ∙ tz = 0.7 ∙ 5 = 3.5 A • h.
The amount of electricity Qp released during discharge is calculated by the formula ηt = Qp / Qz, from where Qp = ηt ∙ Qz = 0.9 ∙ 3.5 = 3.15 A • h.
Discharge time tp = Qp / Ip = 3.15 / 0.3 = 10.5 hours.
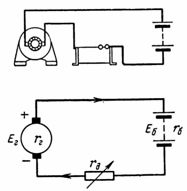
4. The 20 Ah battery was fully charged within 10 hours from the AC mains via a selenium rectifier (Fig. 4). The positive terminal of the rectifier is connected to the positive terminal of the battery when charging. With what current is the battery charged if the current efficiency ηt = 90%? With what current can the battery be discharged within 20 hours?
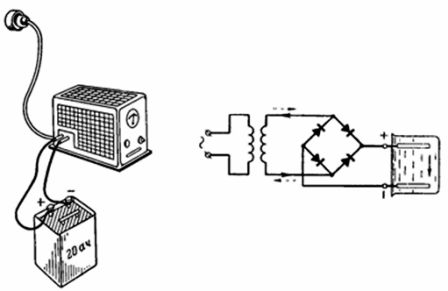
Rice. 4. Figure and diagram for example 4
The battery charging current is: Ic = Q / (ηt ∙ tc) = 20 / (10 ∙ 0.9) = 2.22 A. Allowable discharge current Iр = Q / tr = 20/20 = 1 A.
5. An accumulator battery consisting of 50 cells is charged with a current of 5 A. one battery cell 2.1 V, and its internal resistance rvn = 0.005 Ohm. What is the battery voltage? What is etc. c. must have a charge generator with internal resistance rg = 0.1 Ohm (Fig. 5)?
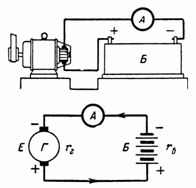
Rice. 5. Figure and diagram for example 5
D. d. C. battery is equal to: Eb = 50 ∙ 2.1 = 105 V.
Internal resistance of the battery rb = 50 ∙ 0.005 = 0.25 Ohm. D. d. S. generator is equal to the sum of e. etc. with batteries and voltage drop in battery and generator: E = U + I ∙ rb + I ∙ rg = 105 + 5 ∙ 0.25 + 5 ∙ 0.1 = 106.65 V.
6. The storage battery consists of 40 cells with internal resistance rvn = 0.005 Ohm and e. etc. p. 2.1 V. The battery is charged with current I = 5 A from the generator, e.g. etc. withwhich is 120 V and the internal resistance rg = 0.12 Ohm. Determine the additional resistance rd, the power of the generator, the useful power of the charge, the power loss in the additional resistance rd and the power loss in the battery (Fig. 6).
Rice. 6. Calculation of the accumulator
Find additional resistance using Kirchhoff's second law:
Eg = Eb + rd ∙ I + rg ∙ I + 40 ∙ rv ∙ I; rd = (Eg-Eb-I ∙ (rg + 40 ∙ rv)) / I = (120-84-5 ∙ (0.12 + 0.2)) / 5 = 34.4 / 5 = 6.88 Ohm …
Since e. etc. c. When the battery is charged, the EMF of the cell at the beginning of charging is 1.83V, then at the beginning of charging, with a constant additional resistance, the current will be more than 5A. In order to maintain a constant charging current, it it is necessary to change the additional resistance.
Power loss in the additional resistance ∆Pd = rd ∙ I ^ 2 = 6.88 ∙ 5 ^ 2 = 6.88 ∙ 25 = 172 W.
Power loss in the generator ∆Pg = rg ∙ I ^ 2 = 0.12 ∙ 25 = 3 W.
Power loss in the internal resistance of the battery ∆Pb = 40 ∙ rvn ∙ I ^ 2 = 40 ∙ 0.005 ∙ 25 = 5 W.
The supplied power of the generator to the external circuit is Pg = Eb ∙ I + Pd + Pb = 84 ∙ 5 + 172 + 5 = 579 W.
Useful charging power Ps = Eb ∙ I = 420 W.