How electroplating is applied at work and at home
In the activities of every competent electrician, there are moments that require an accurate analysis of the phenomena occurring during electrolysis. In many cases, fine-tuning of DC power supplies into operating mode with different timing characteristics is required for full process automation.
Historical reference
For the first time, the basic laws describing the effect of direct current on the behavior of substances dissolved in electrolytes were established by the English scientist Michael Faraday.

Physico-chemical processes of electrolysis take place in an electrolytic cell.
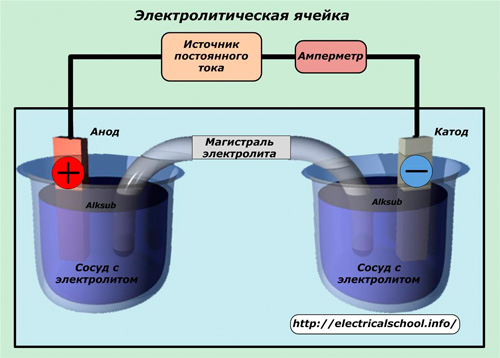
Produced in an electrolyte container. Inside the body are two electrodes to which positive and negative charges are applied from a controlled constant voltage source. The strength of the current flowing through the common circuit is regulated in magnitude and controlled by the operator using meters. Automated electrical cells operate under the supervision of electronics.
The electrode to which the positive charge is applied is called the «anode», and the negative one - the «cathode». Under the action of a current in the electrolyte, ions with charges of opposite signs are formed:
1. cations;
2. anions.
Positively charged ions are called "cations" because they move toward the cathode. Anions are negatively charged ions that are attracted to the anode.
The technologies that arise during electrolysis are at the crossroads of two sciences:
1. chemistry;
2. electrical engineering.
Historically, therefore, the practice has developed that a special branch of electrochemistry deals with galvanization, which studies both the electrochemical and physical phenomena that occur during the deposition of metal cations on any type of anode. This is done in order to choose the optimal technological conditions, to develop special techniques and methods of processing, to choose the nominal modes of equipment during the deposition of certain metals on different bases.
In practice galvanic coating has long been split into two separate, independent directions:
1. electroforming;
2. Galvanizing.
These methods work on approximately the same technologies, but differ in the materials of the base on which the galvanic coating is applied.
Electrotype
This is a way to create a shallow copy of a volume image of a non-metallic part. The main materials can be easily processed plaster, stone, wood, plastic blanks and other substances.
In art workshops, unique forms of jewelry are created by covering leaves from various trees, flowers, insects with a layer of metal.
The founder of galvanic coating was the Russian Boris Semenovich Yakobi, who developed the technology that made it possible to create the famous metal sculptures that still decorate the building of St. Isaac's Cathedral in St. Petersburg. For this work, he received worldwide recognition, was awarded the Demidov Prize, the most prestigious for scientists in Russia, and was awarded a large gold medal during a solemn ceremony at the Paris Exhibition.

The thickness of products created by electroforming methods is characterized by increased dimensions, which give them strength during operation. It can reach from 0.25 to two or more millimeters. This is achieved by the duration of the electrochemical processes.
Non-ferrous precious metals are most often applied by electroplating in artistic products:
-
gold;
-
silver,
-
platinum;
-
rhodium.
For technical purposes use:
-
copper;
-
nickel;
-
iron.
In gilding, silver, nickel plating, copper is used as an intermediate layer in electroplating technological processes.
Galvanizing
This method of electroplating is based on applying a thin layer of protective metal to the surface of a metal part or group of objects. The top cover can perform various functions:
-
corrosion protection;
-
protective decoration;
-
improving the appearance;
-
imparting different electrical properties to the surface in order to improve current conduction or increase insulation characteristics;
-
increasing the strength characteristics of anti-seize;
-
prolongation of wear resistance;
-
improving adhesion when rubberizing steels;
-
increased adhesion to solders and a number of other properties.
A large range of electroplating products can be found in all locations around us.

The above photo shows the processed details that surround us in everyday life: decorated elements of furniture and lamps, protective coatings for household appliances and boxes.
The quality of the layer applied to the product depends on the structure of the created coating. For technical purposes, the most fine-grained and at the same time dense sediment layers are used. They are created:
-
selection of components and electrolyte composition;
-
maintaining an optimal temperature regime of the working environment during electrolysis;
-
current settings, stability of its density and duration of the production cycle.
Types of electroplating
A layer of gold gives the products a rich look, protects against corrosion, increases the reflectivity of the product. The conductive properties of gold-plated surfaces work well in electronic devices.
Silver plating is used for the same purposes and at the same time is often used to improve the conductive properties of power circuits. It is applied to the contacts of starters, contactors, electromagnetic and static relays, legs of operational amplifiers, microcircuits and other electronic components.
Nickel plating allows products made of steel, copper and its alloys, aluminum, zinc and less often tungsten, titanium and molybdenum to give a decorative appearance and provide protection against corrosion not only from atmospheric exposure, but also when working under conditions:
-
contamination with solutions of salts, alkalis, weak acids;
-
increased exposure to mechanical abrasive loads.
Chrome plating increases the hardness and wear resistance of metals and allows you to restore worn surfaces of friction parts to their original parameters. Changing the characteristics of the technology mode allows you to create:
-
matte coatings with a gray tint, which have the greatest hardness, brittleness, but the lowest resistance to wear;
-
shiny surfaces with good wear resistance and hardness;
-
plastic milk coatings with low hardness, but attractive appearance and good anti-corrosion properties. Zinc coating protects steel sheets and steel products from corrosion and is often used in the automotive and construction industries.
Copper coating of steel products protects against corrosion and increases the conductive properties of the metal, used to cover electrical wires operating outdoors.
Brass coating not only protects steel and aluminum alloys from corrosion, but also ensures their good adhesion to the tire.
Armoring gives the surfaces a unique look.
Rhodium plating provides:
-
protecting silver from tarnishing;
-
decorating surfaces;
-
high chemical resistance;
-
increased wear resistance.
Characteristics of technological processes for electroplating
Industrial electroplating methods are widely used in manufacturing processes.

The variety of equipment and methods of applying the outer layer determine a large number of technologies for building surface metal.
Usually technological processes include stages:
1. preliminary preparation of blanks;
2. accumulation of the galvanic layer in the baths;
3. final processing of the part.
At the preliminary stage, mechanical processing and pickling of surfaces is carried out:
-
cleaning from oxides and impurities;
-
preliminary degreasing;
-
attachment to suspended equipment;
-
isolating sites that do not require processing;
-
final degreasing.
During the anodic treatment of the parts, it is important to observe the optimal parameters of the current and their duration.
The final stage includes:
-
neutralization of electrolytic residues on processed parts;
-
alternative treatment with water jets at different temperatures;
-
removal of parts of the suspension elements;
-
removing an isolated layer from closed objects;
-
drying;
-
perform heat treatment, if necessary;
-
mechanical finishing to required size.
Design features of modern electroplating equipment
To accommodate the electrolyte are used galvanic baths made of resistant polymers:
-
PVC;
-
PVDF;
-
polypropylene.
They are mounted on a sturdy metal base together with control units in modular designs.
High-quality cleaning of parts is provided by methods of creation:
-
jet stream;
-
flow method;
-
cascade reception.
The final evaluation of the cleaning process is performed by the operator using visual observation methods.
The installed electrical equipment and heating devices are controlled automatically or by the operator. To speed up operations, bubbling, rocking, and other techniques are performed.
Industrial enterprises are equipped with protective devices, absorbers, on-board suction, wastewater treatment systems and allow only certain processes to be carried out, for example:
-
deposition of nickel-gold layers on industrial products;
-
nickel, silver, copper, chrome plating on pendants;
-
nickel plating in drums;
-
copper and tin processing in small barrels;
-
trim on pendants;
-
wastewater treatment and other technologies.
Industrial plants used in large enterprises are combined into production lines.

Homemade galvanic methods
The use of electroplating and electroplating methods for household purposes is within the power of any home craftsman. However, before making such devices, you should study and take into account the safety rules that must be followed when working with aggressive liquids and electrical installations, ensure good ventilation of the premises and disposal of waste water.
The use of glass tubs is undesirable due to their fragility. It is better to choose dishes made of strong transparent polymers.
For the flow of electric current of constant magnitude in small electrolyte tanks, you can use the design of ready-made blocks from a computer or mobile phone or make them yourself for specific needs.
Quite simple power supply devices from old radios with transistor regulation can be found on the Internet or take the following diagram as a basis.
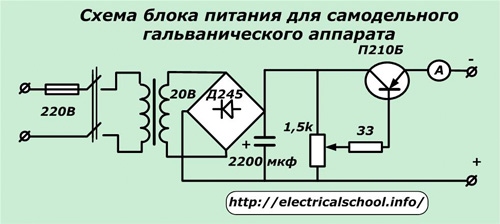
In it, you can use a transformer from any old TV or wind it yourself. The nominal characteristics of the power transistor, the rectifying diode bridge and the regulating resistor are selected according to the power of the load. An electrolytic capacitor equalizes the smoothed voltage. An ammeter is built in for continuous monitoring of the current value.
The arrangement of parts of a similar block, but with an additional node of control transistors, is shown in the photo.
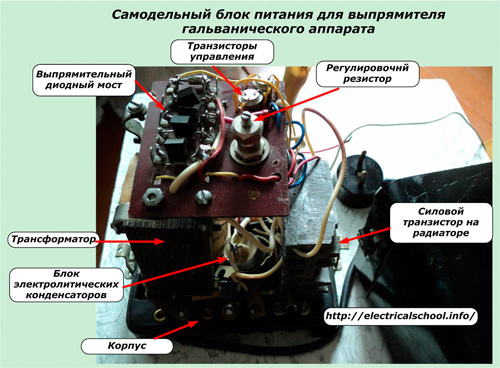
An air cooler is used for better cooling of the power transistor.
It is very easy to make another power supply unit: the outputs of the separate contacts «+» and «-» from the mobile phone charger are connected by a measuring device and a regulating load resistor with the corresponding power to the electrodes of the galvanic bath.

When performing work by galvanic or galvanic methods, a home craftsman will have to independently conduct experiments and record their results in order to gain experience. Only in this way will mastery and practical skills appear.
