Salt baths — device and application
 When heating products in liquid, due to the high values of the coefficient of heat transfer from liquid to metal, a significantly higher heating rate can be achieved. On the other hand, due to the much higher thermal conductivity of liquids compared to gases, the temperature distribution in them must be more uniform, and therefore the heating of individual products or parts of the product will take place under the same conditions.
When heating products in liquid, due to the high values of the coefficient of heat transfer from liquid to metal, a significantly higher heating rate can be achieved. On the other hand, due to the much higher thermal conductivity of liquids compared to gases, the temperature distribution in them must be more uniform, and therefore the heating of individual products or parts of the product will take place under the same conditions.
The fastest heating rate can be achieved in a liquid metal such as molten lead. The lead bath is an iron crucible filled with lead, installed in shaft electric furnace under the exhaust cover. When the lead melts and reaches a predetermined temperature, small parts are lowered into it, which are quickly heated, for example, for quenching or tempering, while the thermal conductivity of the lead ensures a high uniformity of heating of the parts falling into it. but a lead bath has a number of significant defects:
• harmful work with lead, especially at high temperatures,
• impossibility of use for heating to temperatures above 800 ° C (at higher temperatures, lead evaporates intensively),
• low heat capacity of lead, due to which it quickly cools when immersed in larger parts.
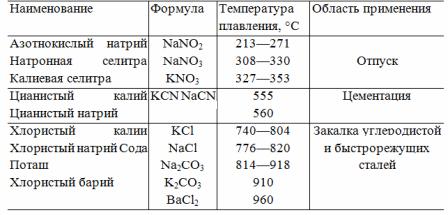
Consequently, lead baths received only limited use. Unlike lead, various salts, nitrates and bases have found much wider application. Since a number of salts, nitrates, and bases used have very different melting points, for any temperature in the range from 250 to 1300 °C such a salt or mixture of salts may be selected as to evaporate little at that temperature and at the same time is fluid. Table 1 gives the melting points and fields of application of some salts and nitrates.
Salt and salt baths constructively performed as baths with external heating, baths with internal heaters and electrodes... The first two types are performed at relatively low temperatures — these are mainly saltpeter and alkaline baths used for heat treatment of profiles and sheets of light alloys (450 -525 °C).
Externally heated salt baths are a rectangular or circular vessel welded from plain carbon steel placed in a shaft with metal heaters.
Salt baths with internal heaters are made the same, but do not have external heating elements, and instead tubular hermetic heating elements are immersed in nitrate. They have significant advantages:
1. Slightly smaller dimensions and lower heat losses compared to external heating baths,
2. the consumption of heating alloys in them is ten times smaller,
3.They are safer because nitrates can explode when overheated in the presence of iron oxides, and such overheating in external heating baths can occur due to contamination of the bottom layers of nitrate, resulting in the bottom of the bath being overheated by the bottom heaters.
The disadvantage of tube heaters in nitrate baths is their short service life due to the high temperature and corrosion of the tube jacket with nitrate.
Table 1. Melting point and range of some salts
Salt and alkaline baths of both types reach very large sizes (length 6-8 m) and a power of several hundred kilowatts. For higher temperatures, baths with an electrode are used. They are a metal or ceramic crucible filled with salt, into which metal electrodes fed by a step-down transformer with a voltage of 8-25 V are lowered.
In a cold state, the salt hardly conducts current, but if it is heated by some external source, then a current is established between the electrodes and releases Joule heat into the salt. Therefore, the molten salt itself serves as a heater in such baths, in which the articles to be heated are immersed.
Electrode baths come with cover and outer electrodes. The former are currently not used due to their low efficiency and uneven heating. In such baths, the current density on the surface of the electrodes due to the large dimensions of the latter is not high, therefore there is only natural thermal circulation of the salt in them, which equalizes the temperatures in the latter along the height. Nevertheless, in such baths the temperature difference in the upper and lower levels can reach 20-25 ° C.
Thus, the main disadvantage of such baths is the insufficiently intensive circulation of the salt, which leads to a decrease in the rate of heating of the products, and therefore in the operation of the bath, and to an uneven distribution of temperature in it along the height.
Moreover, in these baths the current lines fill almost the entire volume of the salt; therefore current also flows through the products. With an unfavorable shape of the latter (sharp edges, thin bridges between two parts of the product), increased current densities can be concentrated in them, which will lead to overheating and can lead to rejection or even melting.
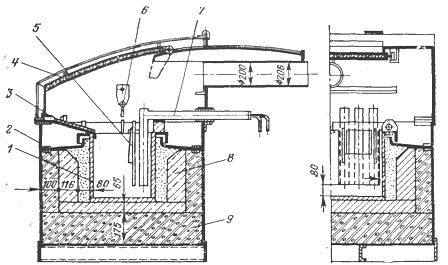
Rice. 1. Salt bath with remote electrodes and partition: 1 — bath, 2 — cladding, 3 — apron, 4 — umbrella, 5 — partition: 6 — pyrometer, 7 — electrode, 8 — refractory masonry, 9 — thermal insulation.
These disadvantages are overcome by electrode salt baths with external electrodes becoming more and more widespread. In them, the electrodes are two rods with a rectangular or circular section, lowered into the salt at a distance of 25-50 mm from each other.
In such baths, almost all current lines are located in the space between two electrodes, therefore only insignificant currents pass through the heated parts and their individual points do not overheat. In addition, in order to completely exclude the passage of current through the parts, the part of the chamber where the electrodes are located can be separated from its working part by a partition (Fig. 1).
Since the current density between the rods is very high, the salt between them is overheated and intense thermal circulation begins, and the heated salt particles rise into the space between the electrodes and at the upper level diverge through the volume of the bath, while colder lower layers insert into the interelectrode space below.
At very high current densities between the electrodes (about 15-25 A / cm2), electromagnetic forces begin to prevail, throwing salt down into the interelectrode space, as a result of which the direction of circulation reverses and its intensity increases. Such forced circulation of salt significantly increases both the coefficient of heat transfer from salt to products and the uniformity of heating of products along the height of the baths (up to ± 3 ° C).
Due to the mentioned advantages, baths with external electrodes have recently been used more and more widely. Salt baths are produced single-phase and three-phase (Fig. 1) at power from 20 to 150 kW and at various temperatures up to 1300 ° C. They are used for heating various products for quenching and tempering and primarily for tools (including high-speed steels), as well as for isothermal annealing. In addition, by choosing the appropriate salt composition in them, it is possible to ensure the conduct of thermochemical processing, carburizing and cyanidation operations of steels.
A well-known advantage of heating in salt baths is to cover items removed from the bath with a thin layer of salt. This film protects the surface of the product from oxidation in the air, while at the same time cracking and rebounding when cooled or when immersed in a cooling tank.
Heat-resistant metal crucibles of electrode baths operating up to 1000 ° C are made of chromium-nickel steels, and their service life can be assumed to be 1 year. Ceramic crucibles can be used up to 1400°C, they can be fully compacted, fired or assembled from individual fired high aluminum ceramic plates bonded together in a solution.
Electrodes can be made of chromium-nickel steels or low-carbon steels, for example class 10. Electrodes stay in high-temperature baths for 3-6 months, in medium-temperature baths for up to one year.
The arrangement of salt bath covers plays an important role... An open salt mirror emits an amount of energy equal to about 5-6 times the heat loss of a closed bath at 1000 ° C. Therefore, the bath cover must be sufficiently insulated, at the same time it must be easy to fold back or move to the side during loading and unloading. A significant reduction in bathroom mirror losses can be achieved by coating its surface with a layer of cell graphite carbon powder.
Since the salt is not conducted in a cold state, it is necessary to warm it to run the bath. The most convenient is the use of initial nichrome resistance. The latter, before the bath solidifies, is immersed in salt and connected to two electrodes. When the bath is heated, the transformer current flowing through the resistance heats it, due to which the layers of salt adjacent to the resistance are heated and, in turn, begin to conduct. The resistor is then turned off and removed from the salt.For such a resistance, a very high specific surface power of the order of 10-15 W / cm2 can be allowed. However, it should be borne in mind that when working in salt, nichrome becomes very fragile and requires careful handling.
Sometimes, instead of a metal resistance between the electrodes, after turning off the furnace, pieces of electrode coal are laid, which, heating up when the bath is turned on, heat the salt. Finally, you can simply heat the salt areas near the electrodes with a gas burner. The operation of heating the bath is quite long, so sometimes it is preferable not to cool the baths overnight, leaving them on at reduced voltage.
In addition to intermittent electrode baths, continuous units are also used... For individual baths, a conveyor belt may be used above the bath to carry the parts and immerse them in the salt. Units for complex heat treatment processes, carried out sequentially in several baths, are more complex, as this requires the creation of alternate movement of parts in horizontal and vertical directions. Usually, this task is solved using a conveyor or a carousel with a lifting device.
Thus, compared to conventional electric furnaces, salt baths have the following advantages:
1. high heating rate and therefore high performance for equal dimensions,
2. easy to perform various types of thermal and thermochemical treatment,
3. protection of products from oxidation during heating and cooling.
The disadvantages of salt baths are as follows:
1.high specific energy consumption due to increased heat losses from the bathroom mirror and the need for its continuous operation due to the duration and complexity of heating (the latter causes underload operation),
2. fairly high consumption of salt,
3. difficult working conditions even with good ventilation.
The prevalence of salt baths is explained by the fact that in many cases their advantages outweigh their disadvantages.
For the lowest temperatures, oil baths are used, performed with both internal and external heating. Electrode boilers for heating water and producing water vapor work in the same way as electrode salt baths.