Production of hydrogen by electrolysis of water — technology and equipment
Electrolysis of water is a physico-chemical process in which water is decomposed into oxygen and hydrogen under the influence of a direct electric current. DC voltage for the cell is obtained, as a rule, by rectification of three-phase alternating current. In an electrolytic cell, distilled water undergoes electrolysis, while the chemical reaction proceeds according to the following well-known scheme: 2H2O + energy -> 2H2 + O2.
As a result of the division of water molecules into parts, hydrogen is obtained by volume twice as much as oxygen. The gases in the plant are dehydrated and cooled before use. The outlet pipes of the device are always protected with non-return valves to prevent fires.
The structure itself is made of steel pipes and thick steel sheets, which gives the whole structure high rigidity and mechanical strength. Gas tanks must be pressure tested.
The electronic unit of the device controls all stages of the production process and allows the operator to monitor the parameters of the panel and pressure gauges, which guarantees safety. The efficiency of electrolysis is such that about 500 cubic meters of both gases are obtained from 500 ml of water at a cost of about 4 kW / h of electrical energy.
Compared to other methods of hydrogen production, water electrolysis has a number of advantages. First, the available raw materials are used — demineralized water and electricity. Second, there are no polluting emissions during production. Third, the process is fully automated. Finally, the output is a fairly pure (99.99%) product.
Therefore, electrolysis plants and the hydrogen produced from them are used today in many industries: in chemical synthesis, in heat treatment of metals, in the production of vegetable oils, in the glass industry, in electronics, in cooling systems in electricity, etc.
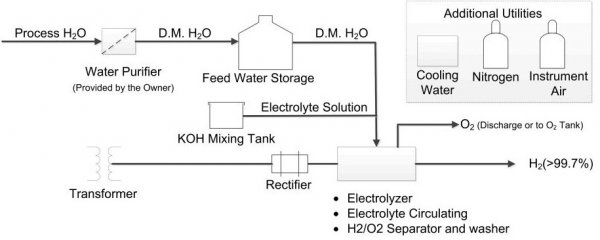
The electrolysis plant is arranged as follows. Outside is the hydrogen generator control panel. In addition, a rectifier, a transformer, a distribution system, a demineralized water system and a block for its replenishment have been installed.
In an electrolytic cell, hydrogen is produced on the cathode plate side and oxygen is produced on the anode side. This is where gases leave the cell. They are separated and fed to a separator, then cooled with demineralized water, then separated by gravity from the liquid phase. The hydrogen is sent to a scrubber where liquid droplets are removed from the gas and cooled in a coil.
Finally, the hydrogen is filtered (filter at the top of the separator), where the water droplets are completely eliminated, and enters the drying chamber. Oxygen is usually directed to the atmosphere. The demineralized water is pumped into the washer.
Here, lye is used to increase the electrical conductivity of the water. If the operation of the electrolyzer continues as usual, then the liquid is topped up once a year in a small amount. Solid potassium hydroxide is placed in a liquid tank two-thirds full of demineralized water, then pumped into solution.
The water cooling system of the electrolyzer serves two purposes: it cools the liquid to 80-90 °C and cools the resulting gases to 40 °C.
The gas analysis system takes hydrogen samples. The drops of lye in the separator are separated, the gas is fed to the analyzer, the pressure is reduced, and the oxygen content of the hydrogen is checked. Before the hydrogen is directed to the tank, the dew point is measured in the hygrometer. A signal will be sent to the operator or to the computer to decide whether the produced hydrogen is suitable for delivery to the storage tank, whether the gas meets the acceptance conditions.
The working pressure of the unit is regulated by an automatic control system. The sensor receives information about the pressure in the electrolyzer, after which the data is sent to a computer, where it is compared with the set parameters. The result is then converted to a signal on the order of 10 mA and the operating pressure is maintained at a predetermined level.

The operating temperature of the unit is regulated by a pneumatic diaphragm valve.The computer will similarly compare the temperature with the setpoint and the difference will be converted into an appropriate signal for PLC.
The safety of the electrolyzer is ensured by the blocking and alarm system. In case of hydrogen leakage, detection is done automatically by detectors. In this case, the program immediately turns off the generation and starts the fan to ventilate the room. The operator should keep a portable leak detector. All these measures make it possible to achieve a high degree of safety in the operation of electrolyzers.