Electrolysis — principle of action, purpose and application
Electrolysis processes
 Electrolysis is widespread in non-ferrous metallurgy and in a number of chemical industries. Metals such as aluminum, zinc, magnesium are obtained mainly by electrolysis. In addition, electrolysis is used to refine (purify) copper, nickel, lead, as well as to produce hydrogen, oxygen, chlorine and a number of other chemicals.
Electrolysis is widespread in non-ferrous metallurgy and in a number of chemical industries. Metals such as aluminum, zinc, magnesium are obtained mainly by electrolysis. In addition, electrolysis is used to refine (purify) copper, nickel, lead, as well as to produce hydrogen, oxygen, chlorine and a number of other chemicals.
The essence of electrolysis is the separation of particles of the substance from the electrolyte when a direct current passes through the electrolytic bath and their deposition on electrodes immersed in the bath (electroextraction) or when substances are transferred from one electrode through the electrolyte to another (electrolytic refining). In both cases, the goal of the processes is to obtain the purest possible substances that are not contaminated with impurities.
In contrast electronic conductivity metals in electrolytes (solutions of salts, acids and bases in water and in some other solvents, as well as in molten compounds), ionic conductivity is observed.
Electrolytes are second class conductors.In these solutions and melts, electrolytic dissociation takes place — the disintegration of positively and negatively charged ions.
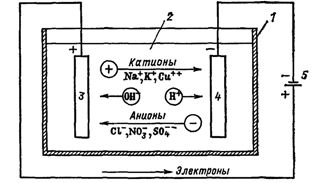
If electrodes connected to a source of electrical energy are placed in a vessel with an electrolyte - an electrolyzer, then an ionic current will begin to flow in it, and positively charged ions - cations will move to the cathode (these are mainly metals and hydrogen), and negatively charged ions — anions (chlorine, oxygen) — to the anode.
At the anode, the anions give up their charge and become neutral particles that settle on the electrode. At the cathode, the cations take electrons from the electrode and are also neutralized, settling on it, and the gases released on the electrodes in the form of bubbles rise up.
Rice. 1. Processes during electrolysis. Electric bath circuit: 1 — bath, 2 — electrolyte, 3 — anode, 4 — cathode, 5 — power supply
The electric current in the external circuit is the movement of electrons from the anode to the cathode (Fig. 1). In this case, the solution is depleted, and in order to maintain the continuity of the electrolysis process, it must be enriched. This is how certain substances are extracted from the electrolyte (electroextraction).

If the electrode is placed in a solution with ions of the same substance from which it is made, then at a certain potential between the electrode and the solution neither the electrode dissolves nor the substance is deposited on it from the solution.
This potential is called the normal potential of the substance. If a more negative potential is applied to the electrode, then the release of a substance (cathodic process) will begin on it, but if it is more positive, then its dissolution will begin (anodic process).
The value of normal potentials depends on ion concentration and temperature. It is generally accepted to consider the normal potential of hydrogen to be zero. Table 1 shows the normal electrode potentials of some aqueous solutions of substances at + 25 ° C.
Table 1. Normal electrode potentials at + 25 ° C
If the electrolyte contains ions of different metals, then ions with a lower negative normal potential (copper, silver, lead, nickel) are separated first at the cathode; alkaline earth metals are the most difficult to isolate. In addition, there are always hydrogen ions in aqueous solutions, which will be released earlier than all metals with a negative normal potential, therefore, during the electrolysis of the latter, a significant or even most of the energy is spent on the release of hydrogen.
With the help of special measures, it is possible to prevent the evolution of hydrogen within certain limits, but metals with a normal potential of less than 1 V (for example, magnesium, aluminum, alkaline earth metals) cannot be obtained by electrolysis from an aqueous solution. They are obtained by decomposition of the molten salts of these metals.
Normal electrode potentials of substances indicated in table.1, are minimal at which the electrolysis process begins, in practice large values of the potential are required for the development of the process.
The difference between the actual potential of an electrode during electrolysis and its normal potential is called overvoltage. It increases energy losses during electrolysis.
On the other hand, increasing the overvoltage for hydrogen ions makes it difficult to release it at the cathode, which makes it possible to obtain by electrolysis from aqueous solutions a number of metals that are more negative than hydrogen, such as lead, tin, nickel, cobalt, chromium and even zinc. This is achieved by conducting the process at increased current densities on the electrodes, as well as by introducing certain substances into the electrolyte.
The course of cathodic and anodic reactions during electrolysis is determined by the following two laws of Faraday.
1. The mass of the substance md released during electrolysis in the cathode or passed from the anode to the electrolyte is proportional to the amount of electricity passed through the electrolyte Azτ: me = α/τ, here a is the electrochemical equivalent of the substance, g / C.
2. The mass of the substance released during electrolysis with the same amount of electricity is directly proportional to the atomic mass of substance A and inversely proportional to its valence n: mNS = A / 96480n, here 96480 is the Faraday number, C x mol-1 .
In this way, the electrochemical equivalent of a substance α= A / 96480n represents the mass of a substance in grams released by a unit amount of electricity passing through the electrolytic bath — a coulomb (ampere-second).
For copper A = 63.54, n =2, α =63.54/96480-2= 0.000329 g / C, for nickel α =0.000304 g / C, for zinc α = 0.00034 g / C

The ratio of the mass of the substance actually released to its mass that should have been released according to Faraday's law is called the current yield of the substance η1.
Therefore, for a real process mNS = η1 NS (A / 96480n) NS It
Naturally, always η1
The current efficiency is significantly dependent on the current density of the electrode. As the electrode current density increases, the current efficiency increases and the process efficiency increases.
The voltage Uel that must be supplied to the electrolyser consists of: breakdown voltage Ep (potential difference of the anodic and cathodic reactions), the sum of the anodic and cathodic overvoltages, the voltage drop in the electrolyte Ep, the voltage drop in the electrolyte Ue = IRep (Rep — electrolytic resistance), voltage drop in tires, contacts, electrodes Uc = I(Rw +Rto +RNS). We get: Uel = Ep + Ep + Ue + Us.
The power consumed during electrolysis is equal to: Rel = IUmail = I(Ep + Ep + Ue + Uc)
Of this power, only the first component is used for conducting reactions, the rest are heat losses of the process. Only during the electrolysis of the molten salts, part of the heat released in the electrolyte IUe is usefully used, since it is spent on melting the salts charged in the electrolyzer.
The efficiency of the electrolysis bath can be estimated by the mass of substance in grams released per 1 J of electricity consumed.This value is called the energy yield of a substance. It can be found by the expression qe = (αη1) /Uel100, here α — electrochemical equivalent of a substance, g / C, η1 — current output, Uemail — voltage of an electrolytic cell, V.