What is measured pressure in physics, pressure units
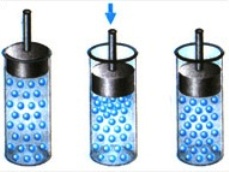
Imagine an air-filled, sealed cylinder with a piston mounted on top. If you start to push the piston, then the volume of air in the cylinder will begin to decrease, the air molecules will collide with each other and with the piston more and more intensively, and the pressure of compressed air on the piston will increase.
If the piston is now suddenly released, the compressed air will push it sharply upward. This will happen because with constant piston area, the force acting on the piston from the compressed air side will increase. The area of the piston remains unchanged, but the force of the gas molecules increases and the pressure increases accordingly.
Or another example. A man is standing on the ground, standing on both feet. In this position, a person is comfortable, does not experience discomfort. But what if that person decides to stand on one leg? He will bend one leg at the knee and now rest on the ground with only one leg. In this position, a person will feel some discomfort, since the pressure on the leg has increased, and about 2 times.Why? Because the area through which the force of gravity now pushes a person to the ground has decreased by 2 times. Here is an example of what pressure is and how easily it can be detected in everyday life.
Physical pressure
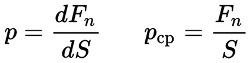
In terms of physics, pressure is a physical quantity that is numerically equal to the force acting perpendicular to the surface per unit area of the given surface. Therefore, to determine the pressure at a certain point on the surface, the normal component of the force applied to the surface is divided by the area of the small surface element on which this force acts. And to determine the average pressure over the entire area, the normal component of the force acting on the surface must be divided by the total area of that surface.
Pascal (Pa)
The pressure is measured in NE in pascals (Pa). This unit of pressure measurement was named in honor of the French mathematician, physicist and writer Blaise Pascal, author of the basic law of hydrostatics - Pascal's Law, which states that the pressure on a liquid or gas is transmitted to any point without changes in all directions. For the first time, the unit of pressure "Pascal" was put into circulation in France in 1961, according to the decree on units, three centuries after the death of the scientist.
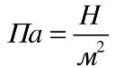
One pascal is equal to the pressure caused by a force of one newton uniformly distributed and directed perpendicular to a surface of one square meter.
Pascals measure not only mechanical pressure (mechanical stress), but also modulus of elasticity, Young's modulus, bulk modulus, yield point, proportional limit, tensile strength, shear resistance, sound pressure, and osmotic pressure. Traditionally, Pascals express the most important mechanical characteristics of materials in a resistant material.
Technical atmosphere (at), physical (atm), kilogram force per square centimeter (kgf / cm2)
In addition to Pascal, other (outside the system) units are used to measure pressure. One of these units is «atmosphere» (c). The pressure in one atmosphere is approximately equal to the atmospheric pressure on the Earth's surface at the level of the World Ocean. Today "atmosphere" is understood as a technical atmosphere (c).

A technical atmosphere (at) is the pressure generated by a force per kilogram (kgf) uniformly distributed over an area of one square centimeter. One kilogram of force, in turn, is equal to the force of gravity acting on a body with a mass of one kilogram under conditions of gravitational acceleration equal to 9.80665 m / s2. Thus, one kilogram of force is equal to 9.80665 newtons, and 1 atmosphere is exactly equal to 98066.5 Pa. 1 at = 98066.5 Pa.
In atmospheres, for example, the pressure in car tires is measured, for example, the recommended pressure in the tires of the passenger bus GAZ-2217 is 3 atmospheres.
There is also a «physical atmosphere» (atm), defined as the pressure of a column of mercury 760 mm high at its base, while the density of mercury is 13,595.04 kg / m3, at a temperature of 0 ° C and under conditions of gravitational acceleration, equal to 9.80665 m / s2.So it turns out that 1 atm = 1.033233 at = 101 325 Pa.
As for the kilogram-force per square centimeter (kgf / cm2), this non-systematic unit of pressure equates with good accuracy to normal atmospheric pressure, which is sometimes convenient for evaluating various effects.
Bar (bar), barium
Outside the system unit «bar» is equal to approximately one atmosphere, but it is more precise - exactly 100,000 Pa. In the SGS system, 1 bar is equal to 1,000,000 dynes / cm2. Previously, the name «bar» was carried by the unit now called «barium» and was equal to 0.1 Pa or in the CGS system 1 barium = 1 dyn / cm2. The words "bar", "barium" and "barometer" come from the same Greek word for "weight".

Often the unit mbar (millibar), equal to 0.001 bar, is used to measure atmospheric pressure in meteorology. And to measure the pressure on planets where the atmosphere is very thin — μbar (microbar), equal to 0.000001 bar. On technical manometers, the scale is most often graduated in bars.
Millimeter of mercury (mmHg), millimeter of water (mmHg)
The non-millimeter of mercury unit is equal to 101325/760 = 133.3223684 Pa. It is denoted "mm Hg", but sometimes it is denoted "torr" - in honor of the Italian physicist, student of Galileo, Evangelista Torricelli, author of the concept of atmospheric pressure.
The unit was created in connection with a convenient way of measuring atmospheric pressure with a barometer, in which the mercury column is in equilibrium under the influence of atmospheric pressure. Mercury has a high density of about 13,600 kg/m3 and has a low saturated vapor pressure at room temperature, which is why mercury was chosen simultaneously for barometers.
At sea level, atmospheric pressure is approximately 760 mm Hg, and it is this value that is now considered normal atmospheric pressure, equal to 101325 Pa or one physical atmosphere, 1 atm. That is, 1 millimeter of mercury is equal to 101325/760 pascals.

In millimeters of mercury, pressure is measured in medicine, meteorology and aviation navigation. In medicine, blood pressure is measured in mm Hg, in vacuum technology pressure measuring instruments are graduated in mmHg along with bars. Sometimes they even write only 25 microns, which means mercury column microns when it comes to evacuation, and pressure measurements are made with vacuum gauges.
In some cases millimeters of water are used and then 13.59 mm water column = 1 mm Hg. Sometimes it is more expedient and convenient. A millimeter of a water column, like a millimeter of a mercury column, is a unit outside the system equal in turn to the hydrostatic pressure of 1 mm of a water column that this column exerts on a flat base at a water column temperature of 4 °C.
