Conductive iron and steel
 In nature, iron is in various compounds with oxygen (FeO, Fd2O3, etc.). It is extremely difficult to isolate chemically pure iron from these compounds. In terms of electrical and magnetic properties, chemically pure iron is close to iron purified from impurities by the electrolytic method (electrolytic iron). The total amount of impurities in electrolytic iron does not exceed 0.03%.
In nature, iron is in various compounds with oxygen (FeO, Fd2O3, etc.). It is extremely difficult to isolate chemically pure iron from these compounds. In terms of electrical and magnetic properties, chemically pure iron is close to iron purified from impurities by the electrolytic method (electrolytic iron). The total amount of impurities in electrolytic iron does not exceed 0.03%.
The main impurities in iron are: oxygen (O2), nitrogen (N2), carbon (C), sulfur (C), phosphorus (P), silicon (Si), manganese (Mn) and some others. Most impurities enter iron from ore and fuel.
Silicon and manganese are specifically introduced into iron as deoxidizers. They easily combine with oxygen and form oxides, which in molten iron (steel) float to the surface in the form of slag and are removed. This improves the mechanical properties of steels, but, remaining in a small amount in the steel, they reduce its electrical conductivity.
Sulfur and phosphorus are harmful impurities. Getting into iron and steel from ore and fuel, they cause embrittlement of steels.Gases (nitrogen and oxygen) are also harmful impurities, as they deteriorate the electrical and magnetic properties of iron and steel.

The technical qualities of iron are low-carbon steels, the carbon content of which varies from 0.01 to 0.1%. In structural steels, carbon is contained in an amount from 0.07 to 0.7%, and in tool and other special (alloy) steels - from 0.7 to 1.7%.
Iron and steel — the cheapest and most accessible conductive materials with high mechanical tensile strength, but their use is limited by the following disadvantages.

To reduce this effect and the magnitude of electrical resistance to alternating current, they try to use steels with the lowest possible magnetic permeability.
For the production of steel wire, steel with a carbon content of 0.10 to 0.15% is used, which has the following properties: density 7.8 g / cm3, melting point 1392 — 1400ОС, maximum tensile strength 55 — 70 kg / mm2, relative elongation 4 — 5%, resistance 0.135 — 146 ohm hmm2 / m, temperature coefficient of resistance α = +0.0057 1 / ° C.
To protect them from atmospheric corrosion, the steel wires are covered with a thin layer of copper or zinc (0.016 — 0.020 mm).
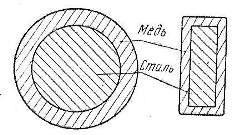
Steel wire and rods are also used as cores in bimetallic wiresproviding significant savings in conductive copper. Bimetallic conductors are used in electrical devices (knife keys, contactors, etc.).
Rice. 1. Cross-section of a bimetallic wire
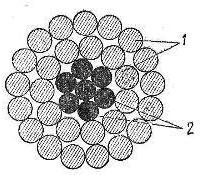
Rice. 2. Cross-section of bimetallic steel-aluminum wire: 1 — aluminum wire, 2 — steel wire
Galvanized steel wire with high mechanical tensile strength (130 — 170 kg / mm2) is used as cores in steel-aluminum wires to increase their mechanical tensile strength.