Metal cleaning and other applications of electrochemical anodic etching
There are a number of methods for the electrochemical treatment of metals to give metal parts the required shape, size or surface layer quality. Such processing is carried out in electrolysers, which are special electrolyte baths, cells, installations and entire machines. In electrochemical anodic etching, local or complete dissolution of the metal occurs by converting its outer layer into an oxide or other easily soluble compound.
Electrochemical etching includes several technologies based directly on the anodic dissolution of the metal. This process is used to clean the surfaces of a wide variety of metal parts, wires, pipes, strips from any oxides, grease and other contaminants, so that you can then apply a high-quality coating, carry out rolling or carry out any other necessary technological process, which requires a chemically clean surface.
During the cleaning of surfaces from contamination by electrochemical etching, this chemical process usually takes place in an acidic solution, usually with the addition of corrosion inhibitors, or in an alkaline solution, or is melted under the action of direct or alternating current in the thickness of the working medium.
Almost any alloy and metal can be electrochemically etched. In this way, electrochemical milling is performed to obtain the desired surface pattern of the metal part by local anodic dissolution of the metal. Areas of the part that must not be left to dissolve are protected with a photoresist layer or chemically resistant pattern prior to etching.
The processing of printed circuit boards, for example, is done in this way. Metal sheets are perforated in the same way and even decorative patterns are made on metal products. Anodic etching can remove jagged or rounded sharp edges on a part.
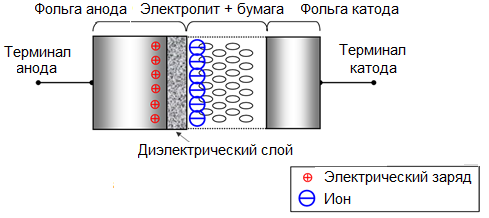
An important field of application of electrochemical etching is to increase the specific surface area of the metal. Etching in chloride solutions of aluminum foil is widely used in the electrical industry for the production of electrolytic capacitors. In this case, the specific working area of the foil increases hundreds of times, and accordingly the specific electrical capacity of the capacitors increases, and the size of the capacitor turns out to be smaller than it could be without processing the foil.
The development of surfaces using electrochemical etching helps to improve the adhesion of metal to ceramic or glass surfaces in electronics, promotes high-quality application of a copy layer on a printing plate in the printing industry, allows you to strengthen the adhesion of enamel to metal when enameling metal products and etc.
In addition, anodic etching helps remove defects galvanic parts for the purpose of their reuse in production, as well as in the recovery of metal plates from offset bimetallic printing plates.
Electrochemical etching is widely used in practical materials science. For example, anodic etching has been successfully used to reveal the microstructure of an alloy in metallographic milling. In this case, the etching takes place under such conditions, when the differences in the dissolution rates of the components of the treated alloy, different in phase and chemical composition, are manifested very sharply.
Selective etching helps reveal phase boundaries, segregation of phosphorus in steel, dendritic structure of titanium alloys, network of cracks in chrome electroplating. Electrochemical etching also makes it possible to assess the susceptibility of stainless steel to intergranular corrosion.

