Galvanic cells and batteries — device, principle of action, types
Low power sources of electrical energy
Galvanic cells and batteries are used to power portable electrical and radio equipment.
Galvanic cells — these are sources of one-time actions, accumulators — reusable action sources.

The simplest galvanic element
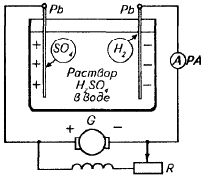
The simplest element can be made of two strips: copper and zinc immersed in water slightly acidified with sulfuric acid. If the zinc is pure enough to have no local reactions, no noticeable change will occur until the copper and zinc are brought together.
However, the strips have a different potential, one with respect to the other, and when connected by a wire, will appear electricity… By this action the zinc strip will gradually dissolve and gas bubbles will form near the copper electrode, collecting on its surface. This gas is hydrogen generated by the electrolyte. Electric current flows from the copper strip along the wire to the zinc strip, and from it through the electrolyte back to the copper.
Gradually, the sulfuric acid of the electrolyte is replaced by zinc sulfate formed from the dissolved part of the zinc electrode. This reduces the voltage of the cell. However, an even greater voltage drop is caused by the formation of gas bubbles on the copper. Both actions cause 'polarization'. Such items have almost no practical value.
Important parameters of galvanic cells
The magnitude of the voltage given by galvanic cells depends only on their type and device, that is, on the material of the electrodes and the chemical composition of the electrolyte, but does not depend on the shape and size of the cells.
The current that a galvanic cell can provide is limited by its internal resistance.
A very important characteristic of the galvanic cell is electrical capacity… Electric capacity means the amount of electricity that a galvanic or storage cell is capable of delivering throughout its operation, that is, until the beginning of final discharge.
The capacity given by the cell is determined by multiplying the strength of the discharge current, expressed in amperes, by the time in hours during which the cell was discharged until the beginning of full discharge. Therefore, capacity is always expressed in ampere-hours (Ah).

By the value of the capacity of the cell, it is also possible to determine in advance how many hours it will work before the start of full discharge. To do this, you need to divide the capacity by the strength of the discharge current permissible for this element.
However, the capacity is not strictly constant. It varies within fairly large limits depending on the operating conditions (mode) of the element and the final discharge voltage.
If the cell is discharged at maximum current and, moreover, without interruptions, it will give a much lower capacity. On the contrary, when the same cell is discharged at a lower current and with frequent and relatively long interruptions, the cell will give up its full capacity.
As for the influence of the final discharge voltage on the cell capacity, it should be borne in mind that during the discharge of the galvanic cell, its operating voltage does not remain at the same level, but gradually decreases.
Common types of electrochemical cells
The most common galvanic cells are manganese-zinc, manganese-air, air-zinc and mercury-zinc systems with salt and alkaline electrolytes. Dry manganese-zinc cells with salt electrolyte have an initial voltage of 1.4 to 1.55 V, the duration of operation at an ambient temperature of -20 to -60 ОFrom 7 to 340 in the morning
Dry zinc-manganese and zinc-air cells with alkaline electrolyte have a voltage of 0.75 to 0.9 V and an operating time of 6 hours to 45 hours.
Dry mercury-zinc cells have a starting voltage of 1.22 to 1.25 V and an operating time of 24 hours to 55 hours.
Dry mercury-zinc cells have the longest guaranteed shelf life of up to 30 months.

Batteries
Batteries These are secondary electrochemical cells. Unlike galvanic cells, no chemical processes take place in the battery immediately after assembly.
In order for the battery to start chemical reactions associated with the movement of electrical charges, it is necessary to appropriately change the chemical composition of its electrodes (and partly of the electrolyte).This change in the chemical composition of the electrodes occurs under the action of an electric current passed through the battery.
Therefore, in order for a battery to produce electric current, it must first be "charged" with direct electric current from some external current source.
Batteries also differ from conventional galvanic cells in the fact that, after discharge, they can be recharged. With good care and under normal operating conditions, batteries can last up to several thousand charges and discharges. Battery powered device
Battery powered device
Currently, lead and cadmium-nickel batteries are most often used in practice. In the first solution of sulfuric acid serves as an electrolyte, and in the second solution of alkali in water. Lead-acid batteries are also called acid, and nickel-cadmium-alkaline batteries.
The principle of operation of batteries is based on the polarization of the electrodes during electrolysis... The simplest acid battery is structured as follows: it is two lead plates immersed in an electrolyte. As a result of the chemical substitution reaction, the plates are covered with a thin coating of lead sulfate PbSO4, as follows from the formula Pb + H2SO4 = PbSO4 + H2.
Acid battery device
This state of the plates corresponds to a discharged battery. If the battery is now turned on for charging, that is, connected to a direct current generator, then the polarization of the plates will begin in it due to electrolysis. As a result of charging the battery, its plates are polarized, i.e. change the substance on their surface and from homogeneous (PbSO4) to different (Pb and PbO2).
The battery becomes the current source, with a plate coated with lead dioxide as the positive electrode and a clean lead plate as the negative electrode.
By the end of charging, the concentration of the electrolyte increases due to the appearance of additional sulfuric acid molecules in it.
This is one of the characteristics of the lead-acid battery: its electrolyte does not remain neutral and itself participates in chemical reactions during battery operation.
By the end of the discharge, both plates of the battery are again covered with lead sulfate, as a result of which the battery ceases to be a source of current. The battery is never brought to this state. Due to the formation of lead sulfate on the plates, the concentration of the electrolyte decreases at the end of the discharge. If the battery is charged, then the polarization can be caused again to put it on discharge again, etc.
How to charge the battery
There are several ways to charge the batteries. The simplest is the normal charging of the battery, which is done as follows. Initially, for 5 — 6 hours, charging is carried out at double normal current until the voltage of each battery reaches 2.4 V.
The normal charging current is determined by the formula Aztax = Q / 16
where Q — nominal capacity of the battery, Ah.
After that, the charging current is reduced to a normal value and charging continues for 15-18 hours until signs of the end of charging appear.
Modern batteries
Nickel-cadmium or alkaline batteries appeared much later than lead batteries, and compared to them are more modern sources of chemical current.The main advantage of alkaline batteries over lead batteries lies in the chemical neutrality of their electrolyte in relation to the active masses of the plates. Therefore, the self-discharge of alkaline batteries is significantly lower than that of lead-acid batteries. The principle of operation of alkaline batteries is also based on the polarization of the electrodes during electrolysis.
To power radio equipment, sealed cadmium-nickel batteries are produced, which are effective at temperatures from -30 to +50 ОC and withstand 400 — 600 charge-discharge cycles. These accumulators are made in the form of compact parallelepipeds and disks weighing from a few grams to kilograms.
Nickel-hydrogen batteries are produced to power autonomous objects. The specific energy of the nickel-hydrogen battery is 50 — 60 Wh kg-1.




