Electrical conductivity of substances
 In this article, we will reveal the topic of electrical conductivity, we will recall what electric current is, how it is related to the resistance of a conductor and, accordingly, to its electrical conductivity. Let's note the main formulas for calculating these quantities, touching on the topic current speed and its relation to electric field strength. We will also touch on the relationship between electrical resistance and temperature.
In this article, we will reveal the topic of electrical conductivity, we will recall what electric current is, how it is related to the resistance of a conductor and, accordingly, to its electrical conductivity. Let's note the main formulas for calculating these quantities, touching on the topic current speed and its relation to electric field strength. We will also touch on the relationship between electrical resistance and temperature.
To begin with, let's recall what electric current is. If you place a substance in an external electric field, then under the action of forces from this field, the movement of elementary charge carriers — ions or electrons — will begin in the substance. It will be an electric shock. The current I is measured in amperes, and one ampere is the current at which a charge equal to one coulomb flows through the cross section of the wire per second.
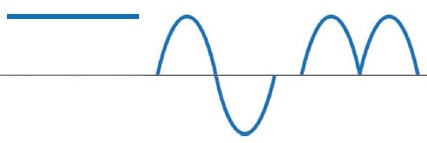
Current is direct, alternating, pulsating.Direct current does not change its magnitude and direction at a given moment, alternating current changes its magnitude and direction over time (AC generators and transformers give exactly alternating current), pulsating current changes its magnitude but does not change direction (eg rectified alternating current) . the current pulses).
Substances tend to conduct an electric current under the action of an electric field, and this property is called electrical conductivity, which is different for different substances. The electrical conductivity of substances depends on the concentration of free charged particles in them, that is, ions and electrons that are not bound neither with the crystal structure, nor with the molecules, nor with the atoms of the given substance. So, depending on the concentration of free charge carriers in a given substance, substances are divided by degree of electrical conductivity into: conductors, dielectrics and semiconductors.
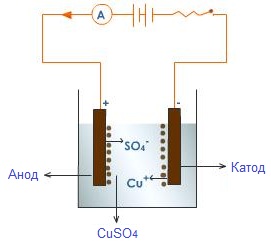
It has the highest electrical conductivity wires of electric current, and by physical nature conductors in nature are represented by two types: metals and electrolytes. In metals, the current is due to the movement of free electrons, that is, they have electronic conductivity, and in electrolytes (in solutions of acids, salts, bases) - from the movement of ions - parts of molecules that have a positive and negative charge, that is, the conductivity of electrolytes is ionic. Ionized vapors and gases are characterized by mixed conductivity, where the current is due to the movement of both electrons and ions.
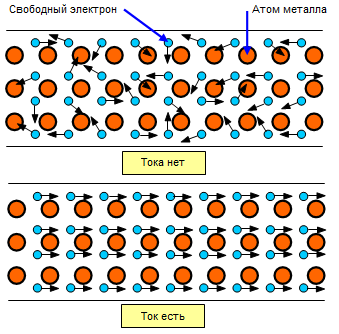
The electron theory perfectly explains the high electrical conductivity of metals.The bond of valence electrons with their nuclei in metals is weak, so these electrons move freely from atom to atom throughout the volume of the conductor.
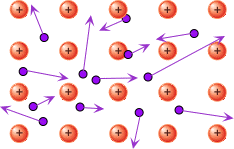
It turns out that the free electrons in metals fill the space between atoms like a gas, an electron gas, and are in chaotic motion. But when a metal wire is introduced into an electric field, the free electrons will move in an orderly manner, they will move towards the positive pole, creating a current. Thus the ordered movement of free electrons in a metal conductor is called an electric current.
It is known that the speed of propagation of an electric field in space is approximately equal to 300,000,000 m / s, that is, the speed of light. This is the same speed at which current flows through a wire.
What does it mean? This does not mean that every electron in the metal moves at such a huge speed, but the electrons in a wire, on the contrary, have a speed of a few millimeters per second to a few centimeters per second, depending on electric field strength, but the speed of propagation of electric current along a wire is exactly equal to the speed of light.
The thing is that each free electron turns out to be in the general electron flow of this same "electron gas", and during the passage of the current, the electric field acts on this entire flow, as a result of which the electrons constantly transmit this field action to each other - from neighbor to neighbor.
But the electrons move to their places very slowly, despite the fact that the speed of propagation of electrical energy along the wire is enormous.So when the switch is turned on in the power plant, current immediately arises throughout the network and the electrons practically stand still.
However, when free electrons move along a wire, they experience many collisions on their way, they collide with atoms, ions, molecules, transferring some of their energy to them. The energy of the moving electrons that overcome this resistance is partially dissipated as heat and the conductor heats up.
These collisions serve as resistance to the movement of electrons, which is why the property of a conductor to prevent the movement of charged particles is called electrical resistance. With a low resistance of the wire, the wire is heated by the current slightly, with a significant one — much stronger and even to white, this effect is used in heating devices and incandescent lamps.
The unit of resistance change is Ohm. Resistance R = 1 ohm is the resistance of such a wire, when a direct current of 1 ampere passes through it, the potential difference at the ends of the wire is 1 volt. The standard of resistance in 1 Ohm is a column of mercury 1063 mm high, cross-section 1 sq. Mm at a temperature of 0 ° C.
Since wires are characterized by electrical resistance, we can say that to some extent the wire is capable of conducting electric current. In this connection, a value called conductivity or electrical conductivity is introduced. Electrical conductivity is the ability of a conductor to conduct an electric current, that is, the reciprocal of the electrical resistance.
The unit of electrical conductivity G (conductivity) is Siemens (S) and 1 S = 1 / (1 Ohm). G = 1 / R.
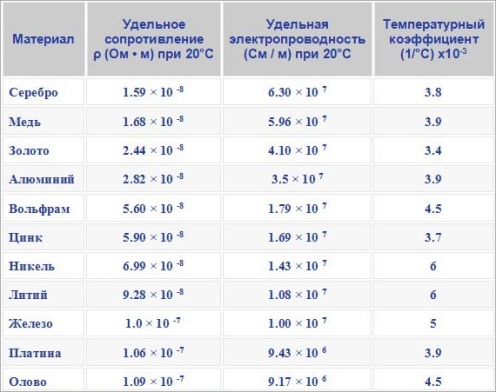
Since the atoms of different substances interfere with the passage of electric current to different degrees, the electrical resistance of different substances is different. For this reason, the concept was introduced electrical resistance, whose value «p» characterizes the conductive properties of this or that substance.
The specific electrical resistance is measured in Ohm * m, that is, the resistance of a cube of substance with an edge of 1 meter. Similarly, the electrical conductivity of a substance is characterized by the specific electrical conductivity ?, measured in S / m, that is, the conductivity of a cube of substance with an edge of 1 meter.
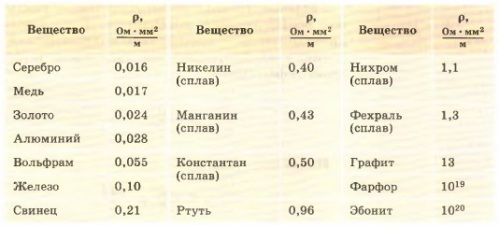
Today, conductive materials in electrical engineering are mainly used in the form of ribbons, tires, wires, with a certain cross-sectional area and a certain length, but not in the form of meter cubes. And for more convenient calculations of electrical resistance and electrical conductivity of wires of specific sizes, more acceptable units of measurement for both electrical resistance and electrical conductivity were introduced. Ohm * mm2 / m — for resistance, and Cm * m / mm2 — for electrical conductivity.
Now we can say that electrical resistance and electrical conductivity characterize the conductive properties of a wire with a cross-sectional area of 1 sq.mm, 1 meter long at a temperature of 20 ° C, it is more convenient.
Metals such as gold, copper, silver, chromium and aluminum have the best electrical conductivity. Steel and iron are less conductive. Pure metals always have better electrical conductivity than their alloys, so pure copper is preferred in electrical engineering.If you need especially high resistance, then tungsten, nichrome, constantan are used.
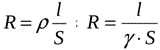
Knowing the value of the specific electrical resistance or electrical conductivity, one can easily calculate the resistance or electrical conductivity of a certain wire made of a given material, taking into account the length l and the cross-sectional area S of this wire.
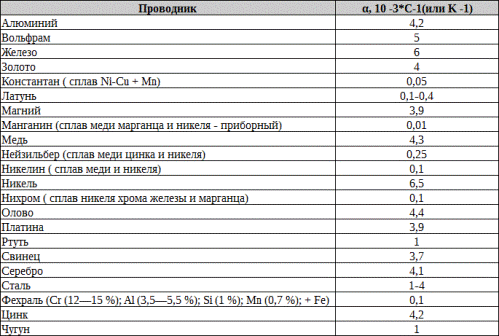
The electrical conductivity and electrical resistance of all materials depend on temperature, because the frequency and amplitude of the thermal vibrations of the atoms of the crystal lattice also increase with increasing temperature, the resistance to electric current and the flow of electrons also increase accordingly.
As the temperature decreases, on the contrary, the vibrations of the atoms of the crystal lattice become smaller, the resistance decreases (electrical conductivity increases). In some substances, the dependence of resistance on temperature is less pronounced, in others it is stronger. For example, such alloys as constantan, fechral and manganin slightly change resistance in a certain temperature range, which is why thermostable resistors are made of them.
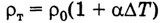
Temperature coefficient of resistance? allows you to calculate for a specific material the increase in its resistance at a certain temperature and numerically characterizes the relative increase in resistance with an increase in temperature by 1 ° C.
Knowing the temperature coefficient of resistance and the temperature rise, it is easy to calculate the resistance of a substance at a given temperature.
 We hope that our article was useful to you and now you can easily calculate the resistance and conductivity of any wire at any temperature.
We hope that our article was useful to you and now you can easily calculate the resistance and conductivity of any wire at any temperature.
