Carriers of electric current
Electricity today is usually defined as "electrical charges and associated electromagnetic fields". The very existence of electric charges is revealed by their strong action on other charges. The space around each charge has special properties: electric forces act in it, which are manifested when other charges are introduced into this space. It's such a space force electric field.
While the charges are stationary, the space between them has properties electric (electrostatic) field… But when the charges are moving, then there are also around them magnetic field… We consider the electric and magnetic field properties separately, but in reality electrical processes are always related to the existence electromagnetic field.

The smallest electric charges are included as components in atom... An atom is the smallest part of a chemical element that carries its chemical properties. An atom is a very complex system. Most of its mass is concentrated in the core. Electrically charged elementary particles revolve around the latter in certain orbits — electrons.
Gravitational forces keep the planets moving around the Sun in orbits, and electrons are attracted to the nucleus of the atom by electrical forces. It is known from experience that only opposite charges attract each other. Therefore, the charges on the nucleus of the atom and the electrons must be different in sign. For historical reasons, it is customary to think of the charge of the nucleus as positive and the charges of the electrons as negative.
Numerous experiments have shown that the electrons of the atoms of each element have the same electric charge and the same mass. At the same time, the electronic charge is elementary, that is, the smallest possible electric charge.
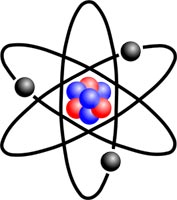
It is customary to distinguish between the electrons located in the inner orbits of the atom and in the outer orbits. The inner electrons are held relatively tightly in their orbits by intraatomic forces. But the outer electrons can relatively easily detach from the atom and remain free for a while or attach to another atom. The chemical and electrical properties of an atom are determined by the electrons in its outer orbits.
The magnitude of the positive charge on the atom's nucleus determines whether the atom belongs to a certain chemical element. An atom (or molecule) is electrically neutral as long as the sum of the negative charges on the electrons equals the positive charge on the nucleus. But an atom that has lost one or more electrons becomes positively charged because of the excess positive charge on the nucleus. It can move under the influence of electrical forces (attractive or repulsive). Such an atom is positive ion… An atom that has captured excess electrons becomes negative ion.
The positive charge carrier in the nucleus of an atom is proton… It is an elementary particle that serves as the nucleus of the hydrogen atom. The positive charge of the proton is numerically equal to the negative charge of the electron, but the mass of the proton is 1836 times the mass of the electron. The nuclei of atoms, in addition to protons, also contain neutrons — particles that have no electrical charge. The mass of a neutron is 1838 times the mass of an electron.
Thus, of the three elementary particles that make up atoms, only the electron and the proton have electric charges. But of these, only the negatively charged electrons can easily move inside the substance, and the positive charges under normal conditions can only move in the form of heavy ions, that is, transfer of the atoms of the substance.
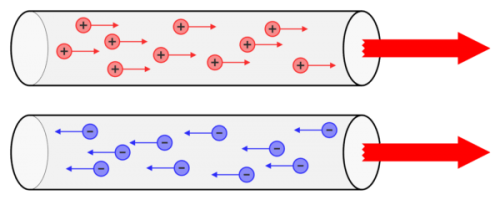
The ordered movement of electric charges is formed, that is, a movement that has a predominant direction in space electricity… Particles whose motion creates an electric current — current carriers in most cases are electrons and much less often — ions.
Allowing for some inaccuracy, it is possible to define current as the directed movement of electric charges. Current carriers can move more or less freely in the substance.
From wires are called substances that conduct current relatively well. All metals are conductors, especially silver, copper, and aluminum.
Conductivity of metals is explained by the fact that in them some of the outer electrons are separated from the atoms. The positive experiments resulting from the loss of these electrons are connected in a crystal lattice — a solid (ionic) skeleton, in the spaces of which there are free electrons in the form of a kind of electron gas.
The smallest external electric field creates a current in the metal, that is, forces the free electrons to mix in the direction of the electric forces acting on them. Metals are characterized by decrease in conductivity with increasing temperature.
Semiconductors conduct electric current much worse than wires. A very large number of substances belong to the number of semiconductors and their properties are very diverse. Electronic conductivity is characteristic of semiconductors (that is, the current in them is created, as in metals, by the directed movement of free electrons - not ions) and, unlike metals, an increase in conductivity with increasing temperature. In general, semiconductors are also characterized by a strong dependence of their conductivity on external influences — radiation, pressure, etc.
Dielectrics (insulators) they practically do not conduct current. An external electric field causes npolarization of atoms, molecules or ions of dielectricsdisplacement under the action of an external field of the elastically bound charges that make up an atom or dielectric molecule. The number of free electrons in dielectrics is very small.
You cannot specify hard boundaries between conductors, semiconductors, and dielectrics. In electrical devices, wires serve as a path for the movement of electrical charges, and dielectrics are needed to properly direct this movement.
Electric current is created due to the action on charges of forces of non-electrostatic origin, called external forces.They create an electric field in the wire, which forces the positive charges to move in the direction of the field forces, and the negative charges, the electrons, in the opposite direction.
It is useful to clarify the concept of translational motion of electrons in metals. Free electrons are in a state of random movement in the space between atoms, in the reverse thermal movement of molecules. The thermal state of the body is caused by collisions of molecules with each other and collisions of electrons with molecules.
The electron collides with molecules and changes the direction of its movement, but gradually continues to move forward, describing a very complex curve. The long-term movement of charged particles in one specific direction, superimposed on their chaotic movement in different directions, is called their drift. Thus, the electric current in metals, according to modern views, is a drift of charged particles.