Why dielectrics do not conduct current
To answer the question «why does a dielectric not conduct electricity?» on the appearance and existence of electric current… And then let's compare how conductors and dielectrics behave in relation to finding an answer to this question.
Current
Electric current is called ordered, that is, directed, movement of charged particles electric field… Thus, first, the existence of an electric current requires the presence of free charged particles capable of moving in a directed manner. Second, an electric field is needed to drive these charges. And, of course, there must be a certain space in which this movement of charged particles, called electric current, takes place.
Free charged particles are abundant in conductors: in metals, in electrolytes, in plasma. In a copper conductor, for example, these are free electrons, in an electrolyte - ions, for example, sulfuric acid ions (hydrogen and sulfur oxide) in a lead-acid battery, in plasma - ions and electrons, they are the ones that move during an electric discharge in an ionized gas.
Metal
For example, let's take two pieces of copper wire and use them to connect a small light bulb to a battery. What will happen? The light will start to glow, which means that a direct electric current… Between the ends of the wires there is now a potential difference created by the battery, which means that an electric field has started to act inside the wire.
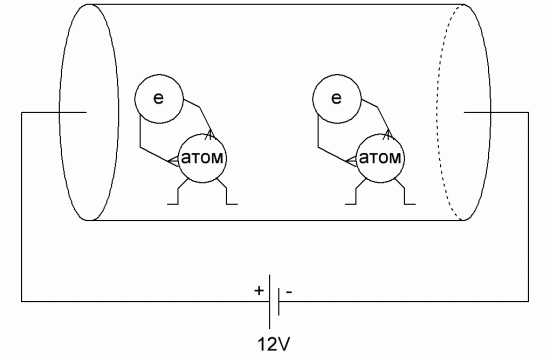
The electric field forces the electrons of the outer shells of copper atoms to move in the direction of the field — from atom to atom, from atom to the next atom, and so on along the chain, because the electrons of the outer shells of metal atoms are much less strongly bound to nuclei than electrons closer to the nuclei of electron orbits. From where the electron was left, another electron comes from the negative terminal of the battery, that is, electrons move freely along the metal chain, easily changing their belonging to atoms.
They seem to form along the crystal lattice of the metal in the direction they are pushed, trying to accelerate, the electric field (from the minus to the plus of the constant EMF source), while the electrons cling to the atoms of the crystal lattice all along their path.
Some electrons in the course of their movement break into atoms (due to the fact that the thermal movement vibrates the entire structure of atoms together with the electrons), as a result of which the conductor heats up - this is how it manifests itself electrical resistance of the wires.
Free electrons in a metal
The study of metals using X-rays, as well as other methods, has shown that metals have a crystalline structure.This means that they consist of atoms or molecules arranged in a certain way in space (in order, ions) that create the correct alternation in all three dimensions.
Under these conditions, the atoms of the elements are located so close to each other that their outer electrons belong to this atom to the same degree as to the neighboring ones, as a result of which the degree of bonding of the electron to each individual atom is practically absent.
Depending on the type of metal, at least one of the electrons of each atom, sometimes two electrons, and in some cases even three electrons are free in terms of their movements in the metal, under the influence of externally imposed forces.

Dielectric
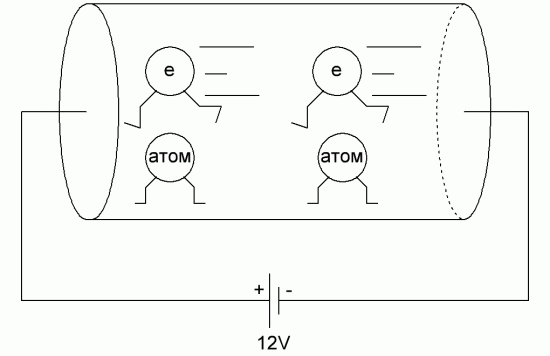
What is in a dielectric? If instead of copper wires you take plastic, paper or something similar? There will be no electricity, no light will come on. Why? The structure of the dielectric is such that it consists of neutral molecules that, even under the action of an electric field, do not release their electrons in an orderly motion — they simply cannot. There are no free conduction electrons in a dielectric, as in a metal.
The outer electrons in the atom of any dielectric molecule are tightly packed, moreover, they participate in the internal bonds of the molecule, while the molecules of such a substance are usually electrically neutral. All dielectric molecules can do is polarize.
Under the action of an electric field applied to them, the associated electric charges of each molecule will simply shift slightly from the equilibrium position, while each charged particle will remain in its own atom. This phenomenon is called charge displacement dielectric polarization.
As a result of polarization, charges appear on the surface of a dielectric polarized in this way by an electric field applied to it, which tend to reduce the external electric field that caused the polarization with their electric field. The ability of a dielectric to weaken an external electric field in this way is called dielectric constant.