The device and principle of operation of the LED
In incandescent lamps, the light comes from a hot-to-white tungsten filament, essentially from heat. Like glowing coals in a furnace, heated by the heating effect of an electric current, when electrons rapidly oscillate and collide with the nodes of the crystal lattice of a conducting metal, at the same time emitting visible light, which, however, represents only less than 15% of the total consumed electrical energy that powers the lamp...
LEDs, unlike incandescent lamps, emit light not because of heat at all, but because of the peculiarity of their design, which is mainly aimed at ensuring that the current energy goes precisely to the emission of light, at a certain wavelength. As a result, the efficiency of the LED as a light source exceeds 50%.
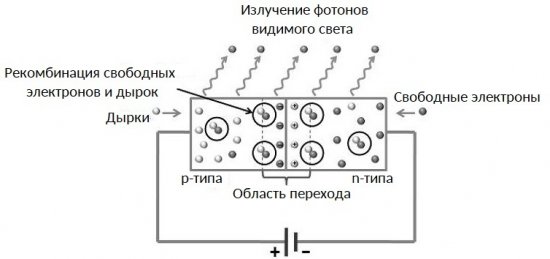
The current flows here across the p-n junction, while in the transition there is a recombination of electrons and holes with the emission of photons (quanta) of visible light of a certain frequency and therefore of a certain color.
Each LED is basically arranged as follows.First, as noted above, there is an electron-hole junction, which consists of p-type semiconductors (the majority of current carriers are holes) and n-type semiconductors that are in contact with each other (more the majority of current carriers are electrons).
When current is transmitted in the forward direction through this junction, then at the point of contact of semiconductors of two opposite types, a charge transition occurs (charge carriers jump between energy levels) from a region of one type of conductivity to a region of a different type of conductivity.
In this case, electrons with their negative charge combine with ions of positively charged holes. At this moment, photons of light are born, the frequency of which is proportional to the difference in the energy levels of the atoms (the height of the potential barrier) between the substances on both sides of the transition.
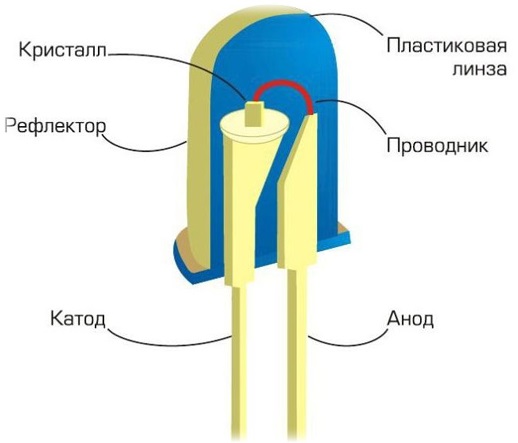
Structurally, LEDs come in a variety of forms. The simplest form is a five millimeter body — a lens. Such LEDs can often be found as indicator LEDs on various household appliances. At the top, the LED housing is shaped like a lens. A parabolic reflector (reflector) is installed in the lower part of the housing.
On the reflector is a crystal that emits light at the point where the current passes through the pn junction. From the cathode — to the anode, from the reflector — in the direction of the thin wire, the electrons move through the cube — the crystal.
This semiconductor crystal is the main element of the LED. Here it is 0.3 by 0.3 by 0.25 mm in size. The crystal is connected to the anode by a thin wire bridge.The polymer body is at the same time a transparent lens that focuses the light in a certain direction, thus obtaining a limited angle of divergence of the light beam.

Today, LEDs come in all colors of the rainbow, from ultraviolet and white to red and infrared. The most common are red, orange, yellow, green, blue and white LED colors. And the color of the glitter here is not determined by the color of the case!
The color depends on the wavelength of the photons emitted by the pn junction. For example, the red color of a red LED has a characteristic wavelength of 610 to 760 nm. The wavelength, in turn, depends on the material that was used in the manufacture of a particular part semiconductor for this LED. So, to get a color from red to yellow, impurities of aluminum, indium, gallium and phosphorus are used.
To obtain colors from green to blue — nitrogen, gallium, indium. To obtain a white color, a special phosphor is added to the crystal, which turns the blue color into white with the help photoluminescence phenomena.
See also: Why should the LED be connected through a resistor