What is electrical conductivity
Speaking about the property of this or that body to prevent the passage of electric current through it, we usually use the term «electrical resistance». In electronics, it is convenient, there are even special microelectronic components, resistors with one or another nominal resistance.
But there is also the concept of "electrical conductivity" or "electrical conductivity", which characterizes the body's ability to conduct an electric current.
Given that resistance is inversely proportional to current, conductivity is directly proportional to current, that is, conductivity is the reciprocal of electrical resistance.
Resistance is measured in ohms and conductivity in siemens. But in fact we are always talking about the same property of the material — its ability to conduct electricity.

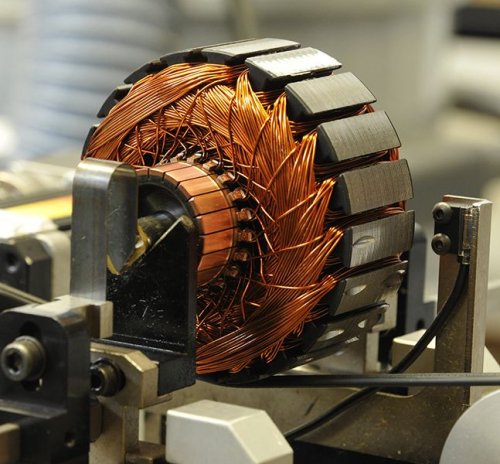
Electronic conductivity suggests that the charge carriers that form the current in matter are electrons. First of all, metals have electronic conductivity, although almost all materials are more or less capable of this.
The higher the temperature of the material, the lower its electronic conductivity, because as the temperature increases, thermal motion increasingly interferes with the orderly motion of electrons and therefore prevents directed current.
The shorter the wire, the larger its cross-sectional area, the greater the concentration of free electrons in it (the lower the specific resistance), the greater the electronic conductivity.
Practically in electrical engineering, it is most important to transmit electrical energy with minimal losses. For that reason metals plays an extremely important role in it. Especially those of them that have the maximum electrical conductivity, that is, the smallest specific electrical resistance: silver, copper, gold, aluminum. The concentration of free electrons in metals is higher than in dielectrics and semiconductors.
It is economically most profitable to use aluminum and copper as conductors of electrical energy from metals, since copper is much cheaper than silver, but at the same time the electrical resistance of copper is only slightly higher than that of silver, respectively the conductivity copper is very little less than silver. Other metals are not as important for the industrial production of wires.
Gaseous and liquid media that contain free ions have ionic conductivity. Ions, like electrons, are charge carriers and can move under the influence of an electric field throughout the volume of a medium. Such an environment can be electrolyte… The higher the temperature of the electrolyte, the higher its ionic conductivity, because with increasing thermal motion, the energy of ions increases and the viscosity of the medium decreases.
In the absence of electrons in the crystal lattice of the material, hole conduction can occur. Electrons carry a charge, but they act like vacancies when the holes move—vacancies in the material's crystal lattice. Free electrons do not move here like a cloud of gas in metals.
Hole conduction occurs in semiconductors on a par with electron conduction. Semiconductors in various combinations allow you to control the amount of conductivity that is demonstrated in various microelectronic devices: diodes, transistors, thyristors, etc.
First of all, metals began to be used as conductors in electrical engineering already in the 19th century, together with dielectrics, insulators (with the lowest electrical conductivity), such as mica, rubber, porcelain.
In electronics, semiconductors have become widespread, occupying an honorable intermediate place between conductors and dielectrics. Most modern semiconductors are based on silicon, germanium, carbon. Other substances are used much less frequently.