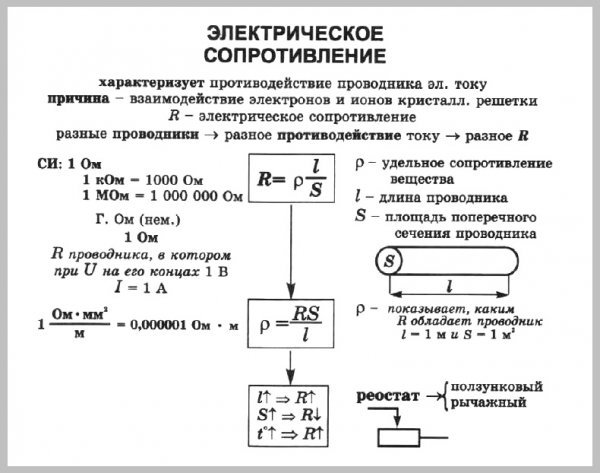
What determines the resistance of a conductor
Resistance and its reciprocal - electrical conductivity - for conductors made of chemically pure metals are a characteristic physical quantity, but nevertheless their resistance values are known with relatively low accuracy.
This is explained by the fact that the resistance value of metals is greatly influenced by various random, difficult to control circumstances.
In the first place, often minor impurities to the pure metal increase its resistance.
The most important metal for electrical engineering is honey, from which wires and cables are made for the distribution of electrical energy, turns out to be particularly sensitive in this regard.
Negligibly small impurities of carbon at 0.05% increase the resistance of copper by 33% compared to the resistance of chemically pure copper, an impurity of 0.13% phosphorus increases the resistance of copper by 48%, 0.5% of iron by 176 %, traces of zinc in an amount difficult to measure due to its smallness, with 20%.
The effect of impurities on the resistance of other metals is less significant than in the case of copper.
The resistance of metals, chemically pure or in general with a certain chemical composition, depends on the method of their thermal and mechanical treatment.
Rolling, drawing, quenching and annealing can change the resistivity of the metal by several percent.
This is explained by the fact that the molten metal crystallizes during solidification, forming numerous and randomly distributed small single crystals.
Any mechanical processing partially destroys these crystals and shifts their groups relative to each other, as a result of which the overall electrical conductivity of a piece of metal usually changes in the direction of increasing resistance.
Prolonged annealing at a favorable temperature, different for different metals, is accompanied by crystal reduction and usually reduces the resistance.
There are methods that make it possible to obtain more or less significant single crystals (single crystals) during solidification of molten metals.
If the metal gives crystals of the correct system, then the resistance of the single crystals of such a metal is the same in all directions. If the metal crystals belong to a hexagonal, tetragonal or trigonal system, then the resistance value of the single crystal depends on the direction of the current.
The limiting (extreme) values are obtained in the direction of the axis of symmetry of the crystal and in the direction perpendicular to the axis of symmetry, in all other directions the resistance has intermediate values.
Pieces of metal obtained by conventional methods, with a random distribution of small crystals, have a resistance equal to a certain average value, unless during solidification a more or less ordered distribution of crystals is established.
From this it is clear that the resistance of samples of other chemically pure metals, the crystals of which do not belong to the correct system, cannot have completely determined values.
Resistance values of the most common conducting metals and alloys at 20 °C: Resistance and electrical conductivity of substances
The influence of temperature on the resistance of various metals is the subject of numerous and thorough studies, since the question of this effect is of great theoretical and practical importance.
Pure metals temperature coefficient of resistance, for the most part is close to the temperature coefficient of thermal linear expansion of gases, i.e. it does not differ much from 0.004, therefore in the range from 0 to 100 ° C the resistance is approximately proportional to the absolute temperature.
At temperatures below 0 ° the resistance decreases faster than the absolute temperature and the faster the temperature decreases. At temperatures close to absolute zero, the resistance of some metals becomes practically zero. At high temperatures above 100 °, the temperature coefficient of most metals increases slowly, i.e. the resistance increases slightly faster than the temperature.
Interesting facts:
The so-called ferromagnetic metals (iron, nickel and cobalt) resistance increases much faster than temperature.Finally, platinum and palladium show an increase in resistivity somewhat lagging behind the increase in temperature.
To measure high temperatures, the so-called platinum resistance thermometer, consisting of a piece of thin pure platinum wire wound spirally over a tube of insulating substance or even fused into the walls of a quartz tube. By measuring the resistance of the wire, you can determine its temperature from a table or from a curve for a temperature range from -40 to 1000 ° C.
Among other substances with metallic conductivity, coal, graphite, anthracite should be noted, which differ from metals with a negative temperature coefficient.
The resistance of selenium in one of its modifications (metallic, crystalline selenium, gray) changes to a significant decrease when exposed to light rays. This phenomenon belongs to the area photovoltaic phenomena.
In the case of selenium and many others like it, the electrons separated from the atoms of the substance when it absorbs light rays do not fly away through the surface of the body, but remain inside the substance, as a result of which the electrical conductivity of the substance naturally increases. The phenomenon is called intrinsic photoelectric phenomenon.
See also:
Why different materials have different resistance
Basic electrical characteristics of wires and cables