Electric current in liquids and gases
Electric current in liquids
 In a metal conductor electricity is formed by the directed movement of free electrons and that no changes occur in the substance of which the conductor is made.
In a metal conductor electricity is formed by the directed movement of free electrons and that no changes occur in the substance of which the conductor is made.
Such conductors, in which the passage of an electric current is not accompanied by chemical changes in their substance, are called first-class conductors... They include all metals, coal and a number of other substances.
But in nature there are also such conductors of electric current in which chemical phenomena occur during the passage of the current. These conductors are called conductors of the second kind... They mainly include various solutions in water of acids, salts and bases.
If you pour water into a glass vessel and add a few drops of sulfuric acid (or some other acid or alkali) to it, and then take two metal plates and attach wires to them, lowering these plates into the vessel, and connect a current source to the other ends of the wires through the switch and the ammeter, then the gas will be released from the solution and it will continue continuously as long as the circuit is closed.acidified water is indeed a conductor. In addition, the plates will begin to become covered with gas bubbles. Then these bubbles will detach from the plates and come out.
When an electric current is passed through the solution, chemical changes occur, resulting in the release of a gas.
They are called conductors of the second type of electrolytes, and the phenomenon that occurs in the electrolyte when an electric current passes through it is electrolysis.
Metal plates immersed in an electrolyte are called electrodes; one of them connected to the positive pole of the current source is called the anode and the other connected to the negative pole is the cathode.
What determines the passage of electric current in a liquid conductor? It turns out that in such solutions (electrolytes) acid molecules (alkalis, salt) under the action of a solvent (in this case water) break up into two components and one part of the molecule has a positive electric charge, and the other a negative one.
Particles of a molecule that have an electrical charge are called ions... When an acid, salt, or alkali is dissolved in water, a large number of both positive and negative ions occur in the solution.
It should now be clear why an electric current passed through the solution, because between the electrodes connected to the current source, a potential differencein other words, one of them turned out to be positively charged and the other negatively charged. Under the influence of this potential difference, positive ions began to mix towards the negative electrode - the cathode, and negative ions - towards the anode.
Thus, the chaotic movement of ions has become an orderly opposite movement of negative ions in one direction and positive ions in the other.This charge transfer process is a flow of electric current through the electrolyte and occurs as long as there is a potential difference across the electrodes. As the potential difference disappears, the current through the electrolyte stops, the ordered movement of ions is disrupted and the chaotic movement begins again.
As an example, consider the phenomenon of electrolysis, when an electric current passes through a solution of copper sulfate CuSO4 with copper electrodes lowered into it.
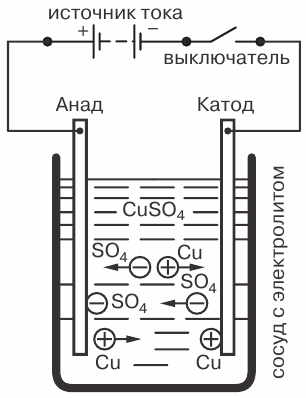
The phenomenon of electrolysis when the current passes through a solution of copper sulfate: C — vessel with electrolyte, B — current source, C — switch
There will also be a reverse movement of ions to the electrodes. The positive ion will be the copper ion (Cu) and the negative ion will be the acid residue (SO4). Copper ions, when in contact with the cathode, will be discharged (attaching the missing electrons to themselves), that is, they will be converted into neutral molecules of pure copper and will be deposited on the cathode in the form of the thinnest (molecular) layer.
Negative ions reaching the anode are also ejected (donate excess electrons). But at the same time, they enter into a chemical reaction with the copper of the anode, as a result of which a copper molecule Cti is added to the acid residue SO4, and a molecule of copper sulfate CnasO4 is formed and returned back to the electrolyte.
Since this chemical process takes a long time, copper is deposited on the cathode, which is released from the electrolyte. In this case, the electrolyte, instead of the copper molecules that went to the cathode, receives new copper molecules due to the dissolution of the second electrode, the anode.
The same process takes place if zinc electrodes are taken instead of copper, and the electrolyte is a solution of zinc sulfate ZnSO4.Zinc will also be transferred from the anode to the cathode.
Therefore, a difference between electric current in metals and liquid conductors lies in the fact that in metals the charge carriers are only free electrons, i.e. negative charges while in electrolytes electricity carried by oppositely charged particles of matter — ions moving in opposite directions. That is why electrolytes are said to have ionic conductivity.

The phenomenon of electrolysis was discovered in 1837 by B. S. Jacobi, who made numerous experiments to study and improve chemical sources of current. Jacobi found that one of the electrodes placed in a solution of copper sulfate, when an electric current passed through it, was coated with copper.
This phenomenon is called electroforming, now it finds an extremely large practical application. One example of this is the coating of metal objects with a thin layer of other metals, for example nickel plating, gold plating, silver, etc.
Electric current in gases
Gases (including air) do not conduct electricity under normal conditions. For example, a goal wires for overhead linesbeing suspended parallel to each other, they are isolated from each other by a layer of air.
However, under the influence of high temperature, a large potential difference and other reasons, gases, like liquid conductors, ionize, that is, particles of gas molecules appear in them in large numbers, which, as carriers of electricity, contribute to the passage of an electric current through the gas.
But at the same time, the ionization of a gas differs from the ionization of a liquid conductor.If the molecule splits into two charged parts in a liquid, then in gases under the action of ionization electrons are always separated from each molecule and the ion remains in the form of a positively charged part of the molecule.
One has only to stop the ionization of the gas, as it ceases to be conductive, while the liquid always remains a conductor of electric current. Therefore, the conductivity of gas is a temporary phenomenon, depending on the action of external causes.

However, there is something else type of electrical dischargeCalled an arc discharge or simply an electric arc. The electric arc phenomenon was discovered at the beginning of the 19th century by the first Russian electrical engineer V. V. Petrov.
V.V. Carrying out numerous experiments, Petrov discovered that between two coals connected to a current source, a continuous electric discharge appeared in the air, accompanied by a bright light. In his writings, V.V. Petrov wrote that in this case "the dark calm can be sufficiently brightly lit." Thus, for the first time, electric light was obtained, which was practically applied by another Russian electrical engineer, Pavel Nikolayevich Yablochkov.
"Svesht Yablochkov", whose work is based on the use of an electric arc, made a real revolution in electrical engineering at that time.

Arc discharge is today used as a light source, for example in spotlights and projection devices. The high temperature of the arc discharge allows it to be used for arc furnace devices… Currently, arc furnaces driven by very high current are used in a number of industries: for melting steel, cast iron, ferroalloys, bronze, etc. And in 1882, NN Benardos first used the arc discharge for cutting and welding metal.
In gas pipes, fluorescent lamps, voltage stabilizers, to obtain electron and ion beams, the so-called glow gas discharge.
Spark discharge Used to measure large potential differences using a spherical spark gap, the electrodes of which are two metal balls with a polished surface. The balls are moved apart and a measurable potential difference is applied to them. The balls are then brought closer together until a spark passes between them. Knowing the diameter of the balls, the distance between them, the pressure, temperature and humidity of the air, they find the potential difference between the balls according to special tables. With this method, it is possible to measure with an accuracy of a few percent the potential difference of the order of tens of thousands of volts.
