Elegas and its properties
SF6 gas — electrical gas — is sulfur hexafluoride SF6 (six fluorine)… SF6 gas is the main insulator in SF6-insulated cell elements.
At working pressure and normal temperatures SF6 gas — colorless, odorless, non-flammable gas, 5 times heavier than air (density 6.7 vs. 1.29 for air), molecular weight also 5 times that of air .
SF6 gas does not age, that is, it does not change its properties over time; it decomposes during an electrical discharge, but quickly recombines, regaining its original dielectric strength.
 At temperatures up to 1000 K, SF6 gas is inert and heat resistant, up to temperatures of about 500 K it is chemically inactive and not aggressive towards the metals used in the construction of SF6 switchgear.
At temperatures up to 1000 K, SF6 gas is inert and heat resistant, up to temperatures of about 500 K it is chemically inactive and not aggressive towards the metals used in the construction of SF6 switchgear.
In an electric field, SF6 gas has the ability to capture electrons, resulting in a high dielectric strength of SF6 gas. By capturing electrons, SF6 gas forms low-mobility ions that are slowly accelerated in an electric field.
Performance of SF6 gas improves in a uniform field, therefore, for operational reliability, the design of individual elements of the switchgear must guarantee the greatest uniformity and homogeneity of the electric field.
In an inhomogeneous field, local overvoltages of the electric field appear, which cause corona discharges. Under the influence of these discharges, SF6 decomposes, forming lower fluorides (SF2, SF4) in the environment, which have a harmful effect on structural materials. complete gas-insulated switchgear (GIS).
To avoid leaks, all surfaces of individual elements of metal parts and grids of cells are clean and smooth and should not have roughness and burrs. The obligation to fulfill these requirements is dictated by the fact that dirt, dust, metal particles also create local stresses in the electric field and thus the dielectric strength of the SF6 insulation deteriorates.
High dielectric strength of SF6 gas allows to reduce the insulation distances at low working pressure of the gas, as a result of which the weight and dimensions of the electrical equipment are reduced. This, in turn, makes it possible to reduce the size of switchgear, which is very important, for example, for the conditions in the north, where each cubic meter of premises is very expensive.
High dielectric strength of SF6 gas provides a high degree of insulation with minimum dimensions and distances, and the good arc extinguishing ability and cooling ability of SF6 increase the breaking capacity of switching devices and reduce heating live parts.
The use of SF6 gas allows, other conditions being equal, to increase the current load by 25% and the permissible temperature of copper contacts up to 90 ° C (in air 75 ° C) due to chemical resistance, non-flammability, fire safety and greater cooling capacity of SF6 gas.
A disadvantage of SF6 is its transition to a liquid state at relatively high temperatures, which sets additional requirements for the temperature regime of the SF6 equipment in operation. The figure shows the dependence of the state of SF6 gas on temperature.
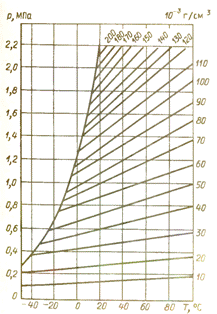
Graph of the state of SF6 gas versus temperature
For the operation of SF6 equipment at negative temperatures minus 40 gr. It is necessary that the pressure of the SF6 gas in the apparatus does not exceed 0.4 MPa at a density of no more than 0.03 g / cm3.
As the pressure increases, the SF6 gas will liquefy at a higher temperature. therefore, in order to improve the reliability of electrical equipment at temperatures of approximately minus 40 ° C, it must be heated (for example, the reservoir of an SF6 circuit breaker is heated to plus 12 ° C to avoid passing SF6 gas into a liquid state).
The arc capacity of SF6 gas, other things being equal, is several times greater than that of air. This is explained by the composition of the plasma and the temperature dependence of the heat capacity, heat and electrical conductivity.
In the plasma state, SF6 molecules disintegrate. At temperatures of the order of 2000 K, the heat capacity of SF6 gas increases sharply due to the dissociation of the molecules. Therefore, the thermal conductivity of plasma in the temperature range 2000 — 3000 K is much higher (by two orders of magnitude) than that of air. At temperatures of the order of 4000 K, the dissociation of molecules decreases.
At the same time, the low ionization potential atomic sulfur formed in the SF6 arc contributes to a concentration of electrons that is sufficient to maintain the arc even at temperatures of the order of 3000 K. As the temperature increases further, the plasma conductivity decreases , reaching the thermal conductivity of air and then increases again. Such processes reduce the voltage and resistance of a burning arc in SF6 gas by 20 — 30% compared to an arc in air to temperatures of the order of 12,000 — 8,000 K. As a result, the electrical conductivity of the plasma decreases.
At temperatures of 6000 K, the degree of ionization of atomic sulfur is significantly reduced and the mechanism of electron capture by free fluorine, lower fluorides and SF6 molecules is enhanced.
At temperatures of about 4000 K, dissociation of molecules ends and recombination of molecules begins, the electron density decreases even more as atomic sulfur chemically combines with fluorine. In this temperature range, the thermal conductivity of the plasma is still significant, the arc is cooled, this is also facilitated by the removal of free electrons from the plasma due to their capture by SF6 molecules and atomic fluorine. The dielectric strength of the gap gradually increases and eventually recovers.
A feature of arc extinguishing in SF6 gas lies in the fact that at a current close to zero, the thin arc rod is still maintained and breaks off at the last moment of the crossing of the current through the zero.In addition, after the current passes through zero, the residual arc column in the SF6 gas cools intensively, including due to the even greater increase in the heat capacity of the plasma at temperatures of the order of 2000 K, and the dielectric strength increases rapidly.
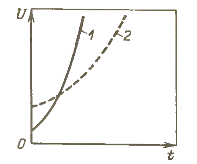
The increase in dielectric strength of SF6 gas (1) and air (2)
Such stability of arc burning in SF6 gas to minimum current values at relatively low temperatures results in the absence of current interruptions and large overvoltages during arc quenching.
In air, the dielectric strength of the gap at the moment the arc current crosses zero is greater, but because of the large time constant of the arc in air, the rate of increase of dielectric strength after the current crosses zero is less .

