How batteries work and work
 In the broadest sense of the word in technology, the term "battery" refers to a device that allows under certain operating conditions to accumulate a certain type of energy, and in others to use it for human needs.
In the broadest sense of the word in technology, the term "battery" refers to a device that allows under certain operating conditions to accumulate a certain type of energy, and in others to use it for human needs.
They are used where it is necessary to collect energy for a certain time and then use it to carry out large labor-intensive processes. For example, hydraulic accumulators used in locks allow ships to rise to a new level on the river bed.
Electric batteries work with electricity on the same principle: first, they accumulate (accumulate) electricity from an external charging source and then give it to connected consumers to do work. By their nature, they belong to chemical current sources capable of carrying out periodic cycles of discharge and charge repeatedly.
During operation, chemical reactions constantly take place between the components of the electrode plates with their filling substance - electrolyte.
A schematic diagram of a battery device can be represented by a simplified drawing when two plates of different metals with wires are inserted into the body of the vessel to provide electrical contacts. An electrolyte is poured between the plates.
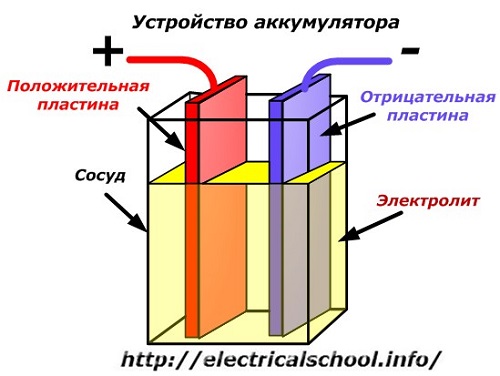
Battery operation when discharged
When a load, such as a light bulb, is connected to the electrodes, a closed electrical circuit is created through which the discharge current flows. It is formed by the movement of electrons in metal parts and anions with cations in the electrolyte.
This process is conventionally shown on a diagram with a nickel-cadmium electrode design.
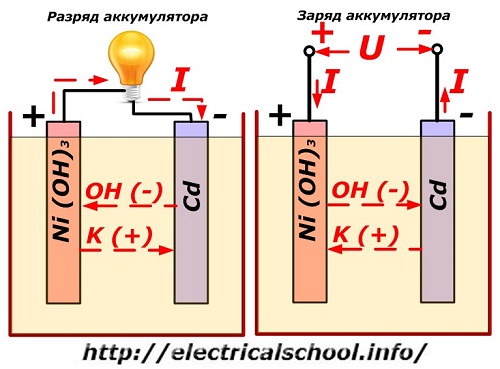
Here, nickel oxides with graphite additives, which increase electrical conductivity, are used as the material of the positive electrode. The metal of the negative electrode is spongy cadmium.
During discharge, active oxygen particles from nickel oxides are released into the electrolyte and directed to the negative plates, where the cadmium is oxidized.
Battery performance when charging
When the load is switched off, a constant (in certain situations, pulsating) voltage is applied to the plate terminals of a greater value than that of a charged battery of the same polarity, when the plus and minus terminals of the source and consumer coincide.
The charger always has more power, which "suppresses" the residual energy in the battery and creates an electric current in the opposite direction of discharge. As a result, the internal chemical processes between the electrodes and the electrolyte change. For example, on a box of nickel-cadmium plates, the positive electrode is enriched with oxygen, and the negative - to a state of pure cadmium.
When the battery is discharged and charged, the chemical composition of the material of the plates (electrodes) changes, but the electrolyte does not change.
Battery connection methods
Parallel connection
The amount of discharge current a person can withstand depends on many factors, but primarily the design, materials used and their dimensions. The larger the area of the plates at the electrodes, the greater the current they can withstand.
This principle is used to connect cells of the same type in parallel in batteries when it is necessary to increase the current to the load. But to charge such a design, it will be necessary to increase the power of the source. This method is rarely used for ready-made structures, since now it is much easier to immediately purchase the necessary battery. But acid battery manufacturers use it, connecting different plates into single blocks.
Serial connection
Depending on the materials used, a voltage of 1.2 / 1.5 or 2.0 volts can be generated between the two electrode plates of batteries common in everyday life. (Actually, this range is much wider.) Obviously, it is not sufficient for many electrical devices. Therefore, batteries of the same type are connected in series, and this is often done in one case.
An example of such a design is the widespread automotive development based on sulfuric acid and lead electrode plates.
Usually, among people, especially among transport drivers, it is customary to call any device a battery, regardless of the number of its constituent elements - boxes. However, this is not entirely correct.The structure, assembled from several boxes connected in series, is already a battery, for which the abbreviated name «АКБ» is affixed... Its internal structure is shown in the figure.
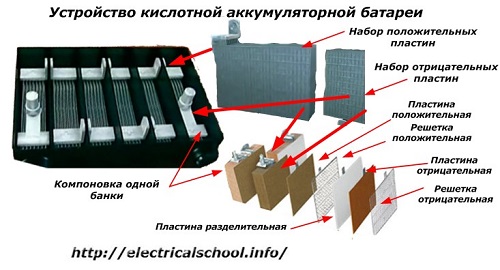
Each of the jars consists of two blocks with a set of plates for the positive and negative electrodes. The blocks fit into each other without metal contact with the possibility of a reliable galvanic connection through the electrolyte.
In this case, the contact plates have an additional grid and are separated from each other by a separator plate.
Connecting the plates in blocks increases their working area, reduces the total resistance of the entire structure and allows you to increase the power of the connected load.
On the outside of the box, such a battery has the elements shown in the figure below.
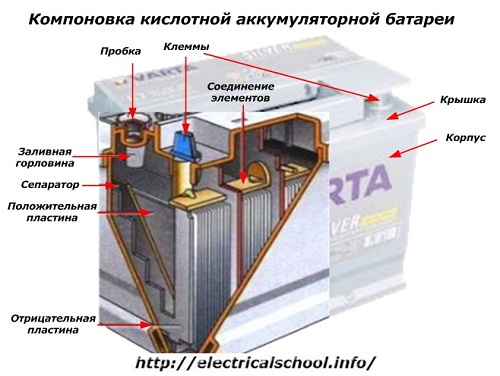
It shows that the sturdy plastic housing is sealed with a cover and equipped with two terminals (usually cone-shaped) on top for connection to the car's electrical circuit. Polarity markings are stamped on their terminals: «+» and «-«. Usually the positive terminal has a slightly larger diameter than the negative terminal to block wiring errors.
Serviceable batteries have a filler hole on the top of each jar to control the electrolyte level or add distilled water during operation. A plug is screwed into it, which protects the internal cavities of the case from contamination and at the same time prevents the electrolyte from spilling when the battery is tilted.
Since with a powerful charge, gassing from the electrolyte is possible (and this process is possible during intensive driving), holes are made in the plugs to prevent the pressure inside the box from increasing.Oxygen and hydrogen, as well as electrolyte vapors, exit through them. It is recommended to avoid such situations involving excessive charging currents.
The same figure shows the connection of the elements between the banks and the arrangement of the electrode plates.
Car starter batteries (lead acid) work on the principle of double sulphation. During discharge / charging, an electrochemical process takes place on them, accompanied by a change in the chemical composition of the active mass of the electrodes with the release / absorption of water in the electrolyte (sulfuric acid).
This explains the increase in the specific gravity of the electrolyte when charging and the decrease when the battery is discharged. In other words, the density value allows you to assess the electrical condition of the battery. A special device is used to measure it - a car hydrometer.
Distilled water, which is part of the electrolyte of acid batteries, turns into a solid state - ice at negative temperatures. Therefore, in order to prevent car batteries from freezing in cold weather, it is necessary to apply special measures provided for in the rules for exploitation.
What types of batteries are there?
Modern production for various purposes produces more than three dozen products with different composition of electrodes and electrolyte. 12 known models run on lithium only.

The following can be found as electrode metal:
-
lead;
-
iron;
-
lithium;
-
titanium;
-
cobalt;
-
cadmium;
-
nickel;
-
zinc;
-
silver;
-
vanadium;
-
aluminum
-
some other items.
They affect the electrical output characteristics and therefore the application.
The ability to withstand short-term high loads resulting from the rotation of the crankshafts of internal combustion engines by electric starter motors is characteristic of lead-acid batteries. They are widely used in transportation, uninterruptible power supplies and emergency power systems.
Standard galvanic cells (regular batteries) are usually replaced by nickel-cadmium, nickel-zinc and nickel-metal hydride batteries.
But lithium-ion or lithium-polymer designs work reliably in mobile and computing devices, construction tools, and even electric vehicles.
According to the type of electrolyte used, the batteries are:
-
sour
-
alkaline.
There is a classification of batteries according to purpose. For example, in modern conditions, devices have appeared that are used for energy transfer — recharging other sources. The so-called external battery helps the owners of many mobile devices in the absence of an alternating electrical network. It is able to repeatedly charge a tablet, smartphone, mobile phone.
All these batteries have the same principle of operation and a similar device. For example, the lithium-ion finger model shown in the figure below in many ways repeats the design of the acid batteries discussed earlier.
Here we see the same contact electrodes, plates, separator and housing. Only they are made taking into account other working conditions.
Basic electrical characteristics of a battery
The operation of the device is affected by the parameters:
-
capacity;
-
energy density;
-
self discharge;
-
temperature regime.
Capacity is called the maximum charge of the battery, which it is able to give during the discharge to the lowest voltage. It is expressed in pendants (SI system) and ampere-hours (non-system unit).
As a type of capacity there is «energy capacity», which determines the energy released during the discharge to the minimum allowable voltage. It is measured in joules (SI) and watt-hours (non-SI units).
Energy density expressed as the ratio of the amount of energy to the weight or volume of the battery.
Self-discharge consider the loss of capacity after charging in the absence of a load on the terminals. This depends on the design and is exacerbated by insulation breakdowns between the electrodes for many reasons.
Operating temperature affects the electrical properties and in case of serious deviations from the norm specified by the manufacturer, it can damage the battery. Heat and cold are unacceptable, they affect the course of chemical reactions and the pressure of the environment inside the box.
