What is dielectric constant
 Every substance or body that surrounds us has certain electrical properties. This is due to the molecular and atomic structure: the presence of charged particles in a mutually bound or free state.
Every substance or body that surrounds us has certain electrical properties. This is due to the molecular and atomic structure: the presence of charged particles in a mutually bound or free state.
When no external electric field acts on the substance, these particles are distributed in such a way that they balance each other and do not create an additional electric field in the entire total volume. In the case of external application of electrical energy inside the molecules and atoms, a redistribution of charges occurs, which leads to the creation of its own internal electric field directed against the external one.
If the vector of the applied external field is denoted as «E0», and the internal one «E '», then the total field «E» will be the sum of the energy of these two quantities.
In electricity, it is customary to divide substances into:
-
wires;
-
dielectrics.
This classification has existed for a long time, although it is quite arbitrary, since many bodies have different or combined properties.
Conductors
Carriers that have free charges are used as conductors.Most often, metals act as conductors, since free electrons are always present in their structure, which are able to move throughout the volume of the substance and at the same time are participants in thermal processes.
When a conductor is isolated from the action of external electric fields, then a balance of positive and negative charges is created in it from ion lattices and free electrons. This equilibrium is immediately destroyed when a conductor in an electric field — due to the energy at which the redistribution of charged particles begins and unbalanced charges with positive and negative values appear on the outer surface.
This phenomenon is usually called electrostatic induction... The charges it charges on the surface of metals are called induction charges.
Inductive charges formed in the conductor form a self-field E ', which compensates for the effect of the external E0 inside the conductor. Therefore, the value of the total, total electrostatic field is compensated and equal to 0. In this case, the potentials of all points both inside and outside are the same.
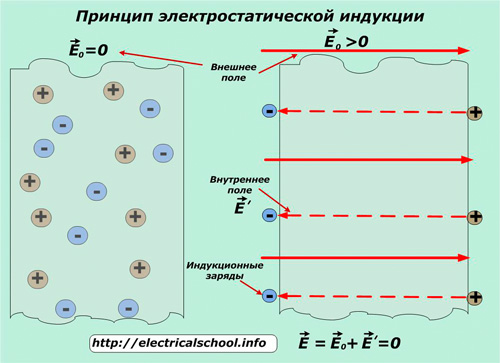
The obtained conclusion shows that inside the conductor, even with an external field connected, there is no potential difference and no electrostatic fields. This fact is used in shielding — the application of a method of electrostatic protection of people and electrical equipment sensitive to induced fields, especially precision measuring instruments and microprocessor technology.
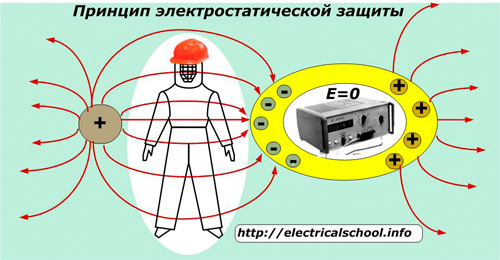
Shielded clothing and footwear made of fabrics with conductive threads, including hats, are used in electricity to protect personnel working in conditions of increased voltage created by high-voltage equipment.
Dielectrics
This is the name of substances that have insulating properties. They only contain interconnected fees, not freebies. They all have positive and negative particles bound in a neutral atom, deprived of freedom of movement. They are distributed inside the dielectric and do not move under the action of the applied external field E0.
However, its energy still causes certain changes in the structure of the substance — inside the atoms and molecules, the ratio of positive and negative particles changes, and on the surface of the substance, excessive, unbalanced associated charges appear, forming an internal electric field E '. It is directed against the tension applied from the outside.
This phenomenon is called dielectric polarization... It is characterized by the fact that an electric field E appears inside the substance, formed by the action of the external energy E0, but weakened by the opposition of the internal E '.
Types of polarization
It is of two types inside dielectrics:
1. orientation;
2. electronic.
The first type has the additional name of dipole polarization. It is inherent in dielectrics with shifted centers at negative and positive charges, which form molecules of microscopic dipoles — a neutral set of two charges. This is characteristic of water, nitrogen dioxide, hydrogen sulfide.
Without the action of an external electric field, the molecular dipoles of such substances are oriented in a chaotic manner under the influence of processes at the operating temperature. At the same time, there is no electric charge at any point of the inner volume and on the outer surface of the dielectric.
This picture changes under the influence of externally applied energy, when the dipoles slightly change their orientation and regions of uncompensated macroscopic bound charges appear on the surface, forming a field E' with a direction opposite to the applied E0.
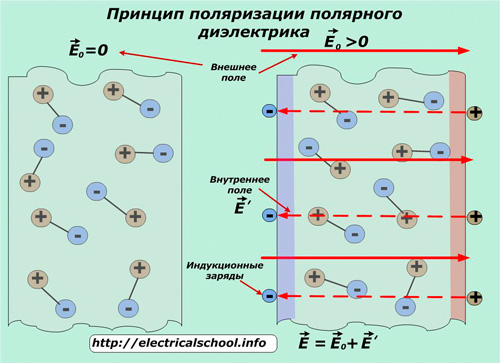
With such polarization, temperature has a great influence on processes, causing thermal motion and creating disorienting factors.
Electronic polarization, elastic mechanism
It manifests itself in non-polar dielectrics — materials of a different type with molecules devoid of a dipole moment, which, under the influence of an external field, are deformed so that the positive charges are oriented in the direction of the E0 vector, and the negative charges are oriented in the opposite direction.
As a result, each of the molecules acts as an electric dipole oriented along the axis of the applied field. In this way, they create on the outer surface their field E 'with the opposite direction.
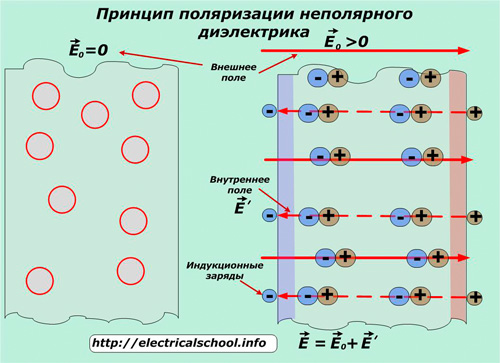
In such substances, the deformation of the molecules and therefore the polarization due to the action of an external field does not depend on their movement under the influence of temperature. Methane CH4 can be cited as an example of a non-polar dielectric.
The numerical value of the internal field of the two types of dielectrics first changes in magnitude in direct proportion to the increase of the external field, and then, when saturation is reached, nonlinear effects appear. They arise when all molecular dipoles are arranged along the lines of force of polar dielectrics or changes have occurred in the structure of non-polar matter, due to the strong deformation of atoms and molecules by large energy applied from the outside.
In practice, such cases are rare — usually failure or failure of the insulation occurs earlier.
The dielectric constant
Among insulating materials, an important role is played by the electrical characteristics and such indicators as the dielectric constant... It can be measured by two different characteristics:
1. absolute value;
2. relative value.
The term absolute dielectric constant substances εa is used when referring to the mathematical notation of Coulomb's law. It, in the form of coefficient εα, connects the vectors of induction D and intensity E.
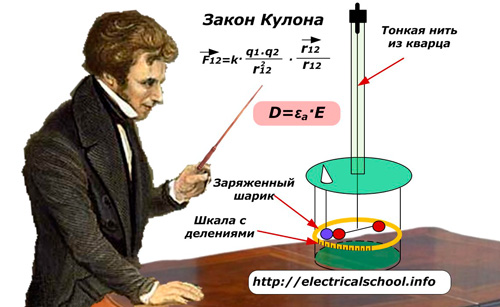
Let us recall that the French physicist Charles de Coulomb, using his own torsion balance, investigated the laws of electric and magnetic forces between small charged bodies.
The determination of the relative permeability of a medium is used to characterize the insulating properties of a substance. It estimates the ratio of the interaction force between two point charges under two different conditions: in vacuum and in a working environment. In this case, the vacuum indices are taken as 1 (εv = 1), while for real substances they are always higher, εr> 1.
The numerical expression εr is shown as a dimensionless quantity explained by the effect of polarization in dielectrics and is used to evaluate their characteristics.
Dielectric constant values of individual media (at room temperature)
Substance ε Substance ε Segnet salt 6000 Diamond 5.7 Rutile (on optical axis) 170 Water 81 Polyethylene 2.3 Ethanol 26.8 Silicon 12.0 Mica 6 Glass beaker 5-16 Carbon dioxide 1.00099 NaCl 5.26 Aqueous steam 1.0126 Benzene 2.322 Air (760 mmHg) 1.00057
