What is dielectric loss and what causes it
 Dielectric losses are the energy dissipated per unit time in a dielectric when an electric field is applied to it and causes the dielectric to heat up. At constant voltage, energy losses are determined only by the strength of the through current due to volume and surface conduction. At alternating voltage, these losses are added to the losses due to different types of polarizations, as well as the presence of semiconductor impurities, iron oxides, carbon, gas inclusions, etc.
Dielectric losses are the energy dissipated per unit time in a dielectric when an electric field is applied to it and causes the dielectric to heat up. At constant voltage, energy losses are determined only by the strength of the through current due to volume and surface conduction. At alternating voltage, these losses are added to the losses due to different types of polarizations, as well as the presence of semiconductor impurities, iron oxides, carbon, gas inclusions, etc.
Considering the simplest dielectric, we can write the expression for the power dissipated in it under the influence of an alternating voltage:
Pa = U·I,
where U is the voltage applied to the dielectric, Aza is the active component of the current flowing through the dielectric.
The dielectric equivalent circuit is usually presented in the form of a capacitor and an active resistance connected in series. From the vector diagram (see Fig. 1):
Aza = Integrated circuit·tgδ,
where δ — the angle between the vector of the total current I and its capacitive component Integrated circuit.
Therefore
Pa = U·Integrated circuit·tgδ,
but the current
Integrated circuit = UΩ C,
where is the capacitance of a capacitor (given dielectric) at angular frequency ω.
As a result, the power dissipated in the dielectric is
Pa = U2Ω C·tgδ,
i.e. the energy losses dissipated in the dielectric are proportional to the tangent of the angle δ which is called dielectric loss angle or simply the angle of loss. This angle δ k characterizes the quality of the dielectric. The smaller the angle di electric losses δ, the higher the dielectric properties of the insulating material.
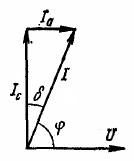
Rice. 1. Vector diagram of currents in a dielectric under alternating voltage.
Introduction of the concept of angle δ It is convenient for practice, because instead of the absolute value of dielectric losses, a relative value is taken into account, which makes it possible to compare insulation products with dielectrics of different quality.
Dielectric losses in gases
Dielectric losses in gases are small. Gases have very low electrical conductivity… The orientation of dipole gas molecules during their polarization is not accompanied by dielectric losses. Addition tgδ=e(U) is called the ionization curve (Fig. 2).
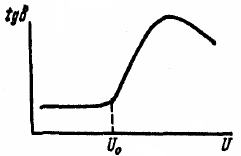
Rice. 2. Change in tgδ as a function of voltage for insulation with air inclusions
A rising tgδ with increasing voltage can assess the presence of gas inclusions in the solid insulation. With significant ionization and losses in the gas, heating and breakdown of the insulation may occur.Therefore, the insulation of the windings of high-voltage electrical machines to remove gas inclusions during production is subjected to a special treatment — drying under vacuum, filling the pores of the insulation with a heated compound under pressure and rolling for pressing.
Ionization of air inclusions is accompanied by the formation of ozone and nitrogen oxides, which have a destructive effect on organic insulation. Ionization of air in uneven fields, for example, in power lines, is accompanied by the effect of visible light (corona) and significant losses, which reduces the transmission efficiency.
Dielectric losses in liquid dielectrics
Dielectric losses in liquids depend on their composition. In neutral (non-polar) liquids without impurities, the electrical conductivity is very low, therefore dielectric losses are also small in them. For example, refined condenser oil has a tgδ
In technology, polar liquids (Sovol, castor oil, etc.) or mixtures of neutral and dipolar liquids (transformer oil, compounds, etc.), in which dielectric losses are significantly higher than those of neutral liquids. For example, the tgδ of castor oil at a frequency of 106 Hz and a temperature of 20°C (293 K) is 0.01.
Dielectric loss of polar liquids depends on viscosity. These losses are called dipole losses because they are due to dipole polarization.
At low viscosity, the molecules are oriented under the action of a frictionless field, the dipole losses in this case are small, and the total dielectric losses are due only to electrical conductivity. Dipole losses increase with increasing viscosity.At a certain viscosity, losses are maximum.
This is explained by the fact that at sufficiently high viscosity the molecules do not have time to follow the change in the field and the dipole polarization practically disappears. In this case, the dielectric losses are small. As the frequency increases, the maximum loss shifts to a higher temperature region.
The temperature dependence of losses is complex: tgδ increases with increasing temperature, reaches its maximum, then decreases to a minimum, then increases again, this is explained by an increase in electrical conductivity. Dipole losses increase with increasing frequency until the polarization has time to follow the change in the field, after which the dipole molecules no longer have time to fully orient themselves in the direction of the field and the losses become constant.
In low-viscosity fluids, conduction losses predominate at low frequencies, and dipole losses are negligible; on the contrary, at radio frequencies the dipole losses are high. Therefore, dipole dielectrics are not used in high frequency fields.
Dielectric losses in solid dielectrics
Dielectric losses in solid dielectrics depend on the structure (crystalline or amorphous), the composition (organic or inorganic) and the nature of the polarization. In such solid neutral dielectrics as sulfur, paraffin, polystyrene, which have only electronic polarization, there are no dielectric losses. Losses can only be due to impurities. Therefore, such materials are used as high-frequency dielectrics.
Inorganic materials, such as single crystals of rock salt, sylvite, quartz, and pure mica, possessing electronic and ionic polarization, have low dielectric losses due to electrical conductivity alone. Dielectric losses in these crystals do not depend on frequency, and tgδ decreases with increasing frequency. As the temperature increases, the losses and tgft change in the same way as the electrical conductivity, increasing according to the law of an exponential function.
In glasses of different composition, for example, ceramics with a high content of the vitreous phase, losses due to electrical conductivity are observed. These losses are caused by the movement of weakly bound ions; they usually occur at temperatures above 50 — 100°C (323 — 373 K). These losses increase significantly with temperature according to the law of an exponential function and depend little on frequency (tgδ decreases with increasing frequency).
In inorganic polycrystalline dielectrics (marble, ceramics, etc.), additional dielectric losses occur due to the presence of semiconductor impurities: moisture, iron oxides, carbon, gas, etc. the same material, because the properties of the material change under the influence of environmental conditions.
Dielectric losses in organic polar dielectrics (wood, cellulose ethers, natural solution, synthetic resins) are due to structural polarization due to loose particle packing. These losses depend on the temperature having a maximum at a certain temperature as well as the frequency increasing with its growth. Therefore, these dielectrics are not used in high frequency fields.
Characteristically, the dependence tgδ on temperature for paper impregnated with the compound has two maxima: the first is observed at negative temperatures and characterizes the loss of fibers, the second maximum at elevated temperatures is due to the loss of the dipole of the compound. As the temperature increases in polar dielectrics, the losses associated with electrical conductivity increase.
