The structure of atoms — elementary particles of matter, electrons, protons, neutrons
 All physical bodies in nature are made of a type of matter called matter. Substances are divided into two main groups — simple and complex substances.
All physical bodies in nature are made of a type of matter called matter. Substances are divided into two main groups — simple and complex substances.
Complex substances are those substances that, through chemical reactions, can be decomposed into other, simpler substances. Unlike complex substances, simple substances are those that cannot be chemically broken down into even simpler substances.
An example of a complex substance is water, which through a chemical reaction can be decomposed into two other, simpler substances - hydrogen and oxygen. As for the last two, they can no longer be chemically decomposed into simpler substances and are therefore simple substances, or, in other words, chemical elements.
In the first half of the 19th century, there was an assumption in science that chemical elements were unchanged substances that had no common relationship with each other. However, the Russian scientist D. I. Mendeleev (1834 — 1907) for the first time in 1869reveals the relationship of chemical elements, showing that the qualitative characteristic of each of them depends on its quantitative characteristic - atomic weight.
Studying the properties of chemical elements, D. I. Mendeleev noticed that their properties periodically repeated depending on their atomic weight. He showed this periodicity in the form of a table, which entered science under the name "Mendeleev's Periodic Table of Elements."
Below is Mendeleev's modern periodic table of chemical elements.
Atoms
According to modern scientific concepts, each chemical element consists of a collection of the smallest material (material) particles called atoms.
An atom is the smallest fraction of a chemical element that can no longer be chemically decomposed into other, smaller and simpler material particles.
Atoms of chemical elements of different nature differ from each other in their physicochemical properties, structure, size, mass, atomic weight, own energy and some other properties. For example, the hydrogen atom differs sharply in its properties and structure from the oxygen atom, and the latter from the uranium atom, and so on.
Atoms of chemical elements are found to be extremely small in size. If we conditionally assume that the atoms have a spherical shape, then their diameters must be equal to hundred millionths of a centimeter. For example, the diameter of a hydrogen atom — the smallest atom in nature — is one hundred millionth of a centimeter (10-8 cm), and the diameter of the largest atoms, for example, the uranium atom, does not exceed three hundred millionths of a centimeter (3 10-8 cm).Therefore, the hydrogen atom is as many times smaller than the sphere of radius one centimeter, as the latter is smaller than the globe.
Due to the very small size of atoms, their mass is also very small. For example, the mass of a hydrogen atom is m = 1.67· 10-24 This means that one gram of hydrogen contains about 6·1023 atoms.
For the conventional unit of measurement of the atomic weights of chemical elements, 1/16 of the weight of an oxygen atom is taken. In accordance with this atomic weight of a chemical element, an abstract number is called, indicating how many times the weight of a given chemical element is more than 1/16 of the weight of an oxygen atom.
In the periodic table of the elements of D. I. Mendeleev, the atomic weights of all chemical elements are given (see the number under the name of the element). From this table we see that the lightest atom is the hydrogen atom, which has an atomic weight of 1.008. The atomic weight of carbon is 12, oxygen is 16, and so on.
As for the heavier chemical elements, their atomic weight exceeds the atomic weight of hydrogen by more than two hundred times. So the atomic value of mercury is 200.6, radium is 226, and so on. The higher the number order occupied by a chemical element in the periodic table of elements, the greater the atomic weight.
Most of the atomic weights of chemical elements are expressed as fractional numbers. This is to some extent explained by the fact that such chemical elements consist of a set of how many types of atoms with different atomic weights but with the same chemical properties.
Chemical elements that occupy the same number in the periodic table of elements and therefore have the same chemical properties but with different atomic weights are called isotopes.
Isotopes are found in most chemical elements, there are two isotopes, calcium - four, zinc - five, tin - eleven, etc. Many isotopes are obtained through art, some of them have great practical importance.
Elementary particles of matter
For a long time, it was believed that the atoms of chemical elements are the limit of the divisibility of matter, that is, as it were, the elementary "building blocks" of the universe. Modern science rejects this hypothesis by establishing that the atom of any chemical element is an aggregate of even smaller material particles than the atom itself.
According to the electron theory of the structure of matter, the atom of any chemical element is a system consisting of a central nucleus around which revolve "elementary" particles of the material called electrons. The nuclei of atoms, according to generally accepted views, consist of a set of "elementary" material particles — protons and neutrons.
In order to understand the structure of atoms and the physico-chemical processes in them, it is necessary to at least briefly familiarize yourself with the basic characteristics of the elementary particles that make up atoms.
It is determined that an electron is a true particle with the smallest negative electric charge observed in nature.
If we conditionally assume that the electron as a particle has a spherical shape, then the diameter of the electron should be equal to 4 ·10-13 cm, that is, it is tens of thousands of times smaller than the diameter of each atom.
An electron, like any other material particle, has mass. The "rest mass" of the electron, that is, the mass it possesses in a state of relative rest, is equal to mo = 9.1 · 10-28 G.
The extremely small "rest mass" of the electron indicates that the inertial properties of the electron are extremely weak, which means that the electron, under the influence of an alternating electric force, can oscillate in space with a frequency of many billions of periods per second.
The mass of the electron is so small that it takes 1027 units to produce one gram of electrons. In order to have at least some physical idea of this colossally large number, we will give an example. If one gram of electrons could be arranged in a straight line close to each other, then they would form a chain four billion kilometers long.
The mass of the electron, like any other material microparticle, depends on the speed of its movement. An electron in a state of relative rest has a "rest mass" of a mechanical nature, similar to the mass of any physical body. As for the "mass of motion" of the electron, which increases as the speed of its motion increases, it is of electromagnetic origin. This is due to the presence of an electromagnetic field in a moving electron as a type of matter with mass and electromagnetic energy.
The faster the electron moves, the more the inertial properties of its electromagnetic field are manifested, the greater is the mass of the latter and, accordingly, its electromagnetic energy. Since the electron with its electromagnetic field represents a single organically connected material system, it is natural the momentum mass of the electron's electromagnetic field to be directly attributed to the electron itself.
The electron, in addition to the properties of a particle, also has wave properties.It was experimentally established that the flow of electrons, like a light flow, propagates in the form of a wave-like movement. The nature of the wave motion of the electron flow in space is confirmed by the phenomena of interference and diffraction of electron waves.
Electronic interference Is the phenomenon of superposition of electron wills on each other and electron diffraction — this is the phenomenon of electron waves bending at the edges of a narrow slit through which the electron beam passes. Therefore, the electron is not just a particle, but a «particle wave», the length of which depends on the mass and speed of the electron.
It was established that the electron, in addition to its translational motion, also performs a rotational motion around its axis. This type of electron movement is called "spin" (from the English word "spin" — spindle). As a result of this movement, the electron, in addition to the electrical properties due to the electric charge, also acquires magnetic properties, resembling in this respect an elementary magnet.
A proton is a real particle with a positive electric charge equal in absolute value to the electric charge of an electron.
The proton mass is 1.67 ·10-24 r, that is, approximately 1840 times greater than the "rest mass" of the electron.
Unlike an electron and a proton, a neutron has no electric charge, that is, it is an electrically neutral "elementary" particle of matter. The mass of the neutron is practically equal to the mass of the proton.
The electrons, protons and neutrons that make up atoms interact with each other. In particular, electrons and protons attract each other as particles with opposite electrical charges.At the same time, electron from electron and proton from proton repel as particles with the same electrical charges.
All these electrically charged particles interact through their electric fields. These fields are a special kind of matter consisting of a collection of elementary material particles called photons. Each photon has a strictly defined amount of energy (energy quantum) inherent in it.
The interaction of particles of electrically charged material materials takes place through the exchange of photons with each other. The force of interaction of electrically charged particles is usually called the electric force.
Neutrons and protons in the nuclei of atoms also interact with each other. However, this interaction between them no longer takes place through an electric field, since the neutron is an electrically neutral particle of matter, but through the so-called nuclear field.
This field is also a special kind of matter consisting of a collection of elementary material particles called mesons... The interaction of neutrons and protons takes place through the exchange of mesons with each other. The force of interaction between neutrons and protons is called the nuclear force.
It has been established that nuclear forces act in the nuclei of atoms at extremely small distances — about 10-13 cm.
Nuclear forces greatly exceed the electrical forces of mutual repulsion of protons in the nucleus of an atom. This leads to the fact that they are able not only to overcome the forces of mutual repulsion of protons inside the nuclei of atoms, but also to create very strong systems of nuclei from the collection of protons and neutrons.
The stability of the nucleus of any atom depends on the ratio of two conflicting forces — nuclear (mutual attraction of protons and neutrons) and electric (mutual repulsion of protons).
Powerful nuclear forces acting in the nuclei of atoms contribute to the transformation of neutrons and protons into each other. These interactions of neutrons and protons take place as a result of the release or absorption of lighter elementary particles, for example mesons.
The particles considered by us are called elementary because they do not consist of an aggregate of other, simpler particles of matter. But at the same time, we must not forget that they are able to transform into each other, to arise at the expense of the other. Thus, these particles are some complex formations, that is, their elementary nature is conditional.
Chemical structure of atoms
The simplest atom in its structure is the hydrogen atom. It consists of a collection of only two elementary particles — a proton and an electron. The proton in the hydrogen atom system plays the role of a central nucleus around which an electron rotates in a certain orbit. In fig. 1 schematically shows a model of the hydrogen atom.
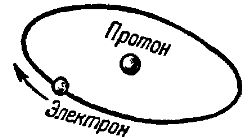
Rice. 1. Diagram of the structure of the hydrogen atom
This model is only a rough approximation of reality. The fact is that the electron as a "wave of particles" does not have a volume sharply demarcated from the external environment. And this means that one should talk not about some exact linear orbit of the electron, but about a kind of electron cloud. In this case, the electron most often occupies some middle line of the cloud, which is one of its possible orbits in the atom.
It should be said that the orbit of the electron itself is not strictly unchanging and stationary in the atom - it also, due to the change in the mass of the electron, makes a certain rotational movement. Therefore, the movement of an electron in an atom is relatively complicated. Since the nucleus of the hydrogen atom (proton) and the electron revolving around it have opposite electrical charges, they attract each other.
At the same time, the free energy of the electron, rotating around the nucleus of the atom, develops a centrifugal force that tends to remove it from the nucleus. Therefore, the electric force of mutual attraction between the nucleus of the atom and the electron and the centrifugal force acting on the electron are opposing forces.
In equilibrium, their electron occupies a relatively stable position in some orbit in the atom. Since the mass of the electron is very small, then in order to balance the force of attraction to the nucleus of the atom, it must spin at an enormous speed equal to about 6·1015 revolutions per second. This means that an electron in the system of a hydrogen atom, like any other atom, moves along its orbit with a linear speed exceeding a thousand kilometers per second.
Under normal conditions, an electron spins in an atom of the kind in the orbit closest to the nucleus. At the same time, it has the minimum possible amount of energy. If for one reason or another, for example, under the influence of other material particles that have invaded the atomic system, the electron moves to an orbit that is more distant from the atom, then it will already have a slightly larger amount of energy.
However, the electron remains in this new orbit for an insignificant amount of time, after which it spins back to the orbit closest to the nucleus of the atom.During this course it gives up its excess energy in the form of a quantum of magnetic radiation—radiant energy (Fig. 2).
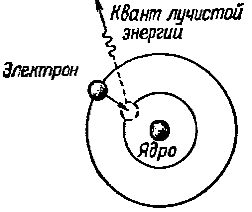
Rice. 2. When an electron moves from a distant orbit to one closer to the nucleus of an atom, it emits a quantum of radiant energy
The more energy the electron receives from the outside, the more it moves into the orbit that is farthest from the nucleus of the atom, and the greater the amount of electromagnetic energy it emits when it spins to the orbit closest to the nucleus.
By measuring the amount of energy emitted by the electron during the transition from different orbits to the one closest to the nucleus of the atom, it was possible to establish that an electron in the system of a hydrogen atom, as in the system of any other atom, cannot went to a random orbit, to a strictly determined in accordance with this energy that it receives under the influence of an external force. The orbits that an electron can occupy in an atom are called allowed orbitals.
Since the positive charge of the nucleus of the hydrogen atom (the charge of the proton) and the negative charge of the electron are numerically equal, their total charge is zero. This means that the hydrogen atom in its normal state is an electrically neutral particle.
This is true for the atoms of all chemical elements: the atom of any chemical element in its normal state is an electrically neutral particle due to the numerical equality of positive and negative charges.
Since the nucleus of a hydrogen atom contains only one "elementary" particle — a proton, the so-called mass number of this nucleus is equal to one. The mass number of the nucleus of an atom of any chemical element is the total number of protons and neutrons that make up that nucleus.
Natural hydrogen consists mainly of a collection of atoms with a mass number equal to one. However, it also contains another type of hydrogen atoms, with a mass number equal to two. The nuclei of these heavy hydrogen atoms, called deuterons, are made up of two particles, a proton and a neutron. This isotope of hydrogen is called deuterium.
Natural hydrogen contains very small amounts of deuterium. For every six thousand light hydrogen atoms (mass number equal to one), there is only one deuterium atom (heavy hydrogen). There is another isotope of hydrogen, super-heavy hydrogen called tritium. In the nucleus of an atom of this hydrogen isotope, there are three particles: a proton and two neutrons, bound together by nuclear forces. The mass number of the nucleus of a tritium atom is three, that is, the tritium atom is three times heavier than the light hydrogen atom.
Although the atoms of hydrogen isotopes have different masses, they still have the same chemical properties, for example, light hydrogen, entering into a chemical reaction with oxygen, forms a complex substance with it - water. Likewise, the isotope of hydrogen, deuterium, combines with oxygen to form water, which, unlike ordinary water, is called heavy water. Heavy water is widely used in the production of nuclear (atomic) energy.
Therefore, the chemical properties of atoms do not depend on the mass of their nuclei, but only on the structure of the electron shell of the atom. Because atoms of light hydrogen, deuterium, and tritium have the same number of electrons (one for each atom), these isotopes have the same chemical properties.
It is not by chance that the chemical element hydrogen occupies the first number in the periodic table of elements.The fact is that there is some relationship between the number of each element in the periodic table of elements and the magnitude of the charge on the nucleus of an atom of that element. It can be formulated as follows: the serial number of each chemical element in the periodic table of elements is numerically equal to the positive charge of the nucleus of that element, and therefore to the number of electrons revolving around it.
Since hydrogen occupies the first number in the periodic table of the elements, this means that the positive charge of the nucleus of its atom is equal to one and that one electron revolves around the nucleus.
The chemical element helium is second in the periodic table of elements. This means that it has a positive electric charge of the nucleus equal to two units, that is, its nucleus must contain two protons, and in the electron shell of the atom - two electrodes.
Natural helium consists of two isotopes — heavy and light helium. The mass number of heavy helium is four. This means that in addition to the two protons mentioned above, two more neutrons must enter the nucleus of the heavy helium atom. As for the light helium, its mass number is three, that is, in addition to two protons, one more neutron should enter the composition of its nucleus.
It has been found that in natural helium the number of light helium atoms is approximately one millionth of the heavy gen atoms. In fig. 3 shows a schematic model of the helium atom.
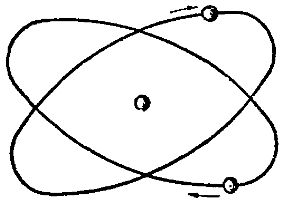
Rice. 3. Diagram of the structure of the helium atom
The further complication of the structure of atoms of chemical elements is due to an increase in the number of protons and neutrons in the nuclei of these atoms and simultaneously to an increase in the number of electrons rotating around the nuclei (Fig. 4). Using the periodic table of elements, it is easy to determine the number of electrons, protons and neutrons that make up different atoms.
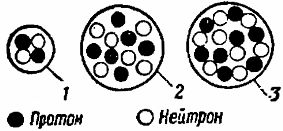
Rice. 4. Schemes of the construction of atomic nuclei: 1 — helium, 2 — carbon, 3 — oxygen
The regular number of a chemical element is equal to the number of protons in the nucleus of the atom and at the same time the number of electrons revolving around the nucleus. As for the atomic weight, it is approximately equal to the mass number of the atom, that is, the number of protons and neutrons taken together in the nucleus. Therefore, by subtracting from the atomic weight of an element a number equal to the element's atomic number, it is possible to determine how many neutrons are contained in a given nucleus.
It has been established that the nuclei of light chemical elements, which have an equal number of protons and neutrons in their composition, are distinguished by very high strength, since the nuclear forces in them are relatively large. For example, the nucleus of a heavy helium atom is extremely durable because it consists of two protons and two neutrons bound together by powerful nuclear forces.
The nuclei of the atoms of heavier chemical elements already contain in their composition an unequal number of protons and neutrons, which is why their bond in the nucleus is weaker than in the nuclei of light chemical elements. The nuclei of these elements can be split relatively easily when bombarded with atomic "projectiles" (neutrons, helium nuclei, etc.).
As for the heaviest chemical elements, especially the radioactive ones, their nuclei are characterized by such low strength that they spontaneously disintegrate into their component parts. For example, atoms of the radioactive element radium, consisting of a combination of 88 protons and 138 neutrons, spontaneously decay, becoming atoms of the radioactive element radon. The atoms of the latter, in turn, break up into their constituent parts, passing into the atoms of other elements.
Having briefly familiarized ourselves with the constituent parts of the nuclei of atoms of chemical elements, let us consider the structure of the electron shells of atoms. As you know, electrons can revolve around the nuclei of atoms only in strictly defined orbits. Moreover, they are so clustered in the electron shell of each atom that individual electron shells can be distinguished.
Each shell can contain a certain number of electrons, which do not exceed a strictly certain number. So, for example, in the first electron shell closest to the nucleus of an atom there can be a maximum of two electrons, in the second - no more than eight electrons, etc.
Those atoms in which the outer electron shells are completely filled have the most stable electron shell. This means that an atom firmly holds all its electrons and does not need to receive an additional amount of them from the outside. For example, a helium atom has two electrons completely filling the first electron shell, and a neon atom has ten electrons, of which the first two completely fill the first electron shell and the rest - the second (Fig. 5).
Rice. 5. Diagram of the structure of the neon atom
Therefore, helium and neon atoms have fairly stable electron shells, they do not tend to change them in any quantitative way. Such elements are chemically inert, that is, they do not enter into chemical interaction with other elements.
However, most chemical elements have atoms where the outer electron shells are not completely filled with electrons. For example, a potassium atom has nineteen electrons, eighteen of which completely fill the first three shells, and the nineteenth electron is in the next, unfilled electron shell. The weak filling of the fourth electron shell with electrons leads to the fact that the nucleus of the atom very weakly holds the outermost - the nineteenth electron, and therefore the latter can be easily removed from the atom. …
Or, for example, the oxygen atom has eight electrons, two of which completely fill the first shell, and the remaining six are located in the second shell. Thus, for the complete completion of the construction of the second electron shell in the oxygen atom, it lacks only two electrons. Therefore, the oxygen atom not only firmly holds its six electrons in the second shell, but also has the ability to attract two missing electrons to itself to fill its second electron shell. This he achieves by chemical combination with the atoms of such elements in which the outer electrons are weakly associated with their nuclei.
Chemical elements whose atoms do not have outer electron layers completely filled with electrons are, as a rule, chemically active, that is, they willingly enter into a chemical interaction.
So, the electrons in the atoms of the chemical elements are arranged in a strictly defined order, and any change in their spatial arrangement or quantity in the electron shell of the atom leads to a change in the physico-chemical properties of the latter.
The equality of the number of electrons and protons in the atomic system is the reason why its total electric charge is zero. If the equality of the number of electrons and protons in the atomic system is violated, then the atom becomes an electrically charged system.
An atom in the system of which the balance of opposite electric charges is disturbed due to the fact that it has lost part of its electrons or, conversely, has acquired an excess of them, is called an ion.
On the contrary, if an atom acquires any excess number of electrons, it becomes a negative ion. For example, a chlorine atom that has received one additional electron becomes a singly charged negative chlorine ion Cl-... An oxygen atom that has received two additional electrons becomes a doubly charged negative oxygen ion O, and so on.
An atom that has become an ion becomes an electrically charged system with respect to the external environment. And this means that the atom began to possess an electric field, together with which it forms a single material system, and through this field it carries out electrical interaction with other electrically charged particles of matter — ions, electrons, positively charged nuclei of atoms, etc.
The ability of different ions to attract each other is the reason they combine chemically, forming more complex particles of matter - molecules.
In conclusion, it should be noted that the dimensions of the atom are very large compared to the dimensions of the real particles of which they are composed. The nucleus of the most complex atom, together with all the electrons, occupies one billionth of the volume of the atom. A simple calculation shows that if one cubic meter of platinum can be pressed so tightly that the intra-atomic and inter-atomic spaces disappear, then a volume equal to about one cubic millimeter will be obtained.
