Dielectric strength
Dielectric strength determines the ability of a dielectric to withstand an electrical voltage applied to it. So, the electrical strength of the dielectric is understood as the average value of the electric field strength Epr at which an electrical breakdown occurs in the dielectric.
The electrical breakdown of a dielectric is a phenomenon of a sharp increase in the electrical conductivity of a given material under the action of a voltage applied to it, with the subsequent formation of a conductive plasma channel.
An electrical breakdown in liquids or gases is also called an electrical discharge. In fact, such a discharge is formed capacitor discharge currentformed by electrodes to which a breakdown voltage is applied.
In this context, the breakdown voltage Upr is the voltage at which electrical breakdown begins, and therefore the dielectric strength can be found using the following formula (where h is the thickness of the sample to be broken down):
Epr = UNC/h
Obviously, the breakdown voltage in any particular case is related to the dielectric strength of the considered dielectric and depends on the thickness of the gap between the electrodes.Accordingly, as the gap between the electrodes increases, the breakdown voltage value also increases. In liquid and gaseous dielectrics, the development of the discharge during breakdown occurs in different ways.

Dielectric strength of gaseous dielectrics
Ionization — the process of converting a neutral atom into a positive or negative ion.
In the process of breaking down a large gap in a gas dielectric, several stages follow one after the other:
1. A free electron appears in the gas gap as a result of photoionization of a gas molecule, directly from a metal electrode or accidentally.
2. The free electron appearing in the gap is accelerated by the electric field, the energy of the electron increases and eventually becomes sufficient to ionize a neutral atom upon collision with it. That is, impact ionization occurs.
3. As a result of many impact ionization actions, an electron avalanche forms and develops.
4. A streamer is formed — a plasma channel formed by positive ions left after the passage of an avalanche of electrons, and negative ones, which are now drawn into the positively charged plasma.
5. Capacitive current through the streamer causes thermal ionization and the streamer becomes conductive.
6. When the discharge gap is closed by the discharge channel, the main discharge occurs.
If the discharge gap is small enough, then the breakdown process can end already at the stage of avalanche breakdown or at the stage of streamer formation - at the stage of the spark.
The electrical strength of gases is determined by:
-
Distance between electrodes;
-
Pressure in the gas to be drilled;
-
The affinity of gas molecules for an electron, the electronegativity of a gas.
The pressure relationship is explained as follows. As the pressure in the gas increases, the distances between its molecules decrease. During the acceleration, the electron must acquire the same energy with a much shorter free path, which is enough to ionize an atom.
This energy is determined by the speed of the electron during the collision, and the speed develops due to acceleration from the force acting on the electron from the electric field, that is, due to its strength.
The Paschen curve shows the dependence of the breakdown voltage Upr in gas on the product of the distance between the electrodes and the pressure — p * h. For example, for air at p * h = 0.7 Pascal * meter, the breakdown voltage is about 330 volts. The increase in breakdown voltage to the left of this value is due to the fact that the probability of an electron colliding with a gas molecule decreases.
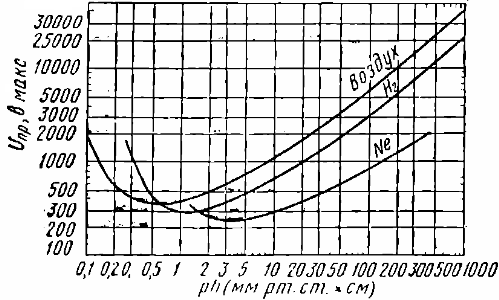
Electron affinity is the ability of some neutral molecules and gas atoms to attach additional electrons to themselves and become negative ions. In gases with high electron affinity atoms, in electronegative gases the electrons need a large accelerating energy to form an avalanche.
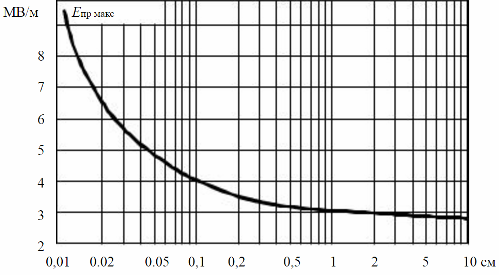
It is known that under normal conditions, that is, at normal temperature and pressure, the dielectric strength of air in a gap of 1 cm is approximately 3000 V / mm, but at a pressure of 0.3 MPa (3 times more than usual) the dielectric strength of the same air becomes close to 10,000 V / mm. For SF6 gas, an electronegative gas, the dielectric strength under normal conditions is approximately 8700 V/mm. And at a pressure of 0.3 MPa, it reaches 20,000 V / mm.
Dielectric strength of liquid dielectrics
As for liquid dielectrics, their dielectric strength is not directly related to their chemical structure. And the main thing that affects the mechanism of decay in a liquid is the very close, compared to a gas, arrangement of its molecules. Impact ionization, characteristic of gases, is impossible in a liquid dielectric.
The impact ionization energy is approximately 5 eV, and if we express this energy as the product of the electric field strength, the electron charge, and the mean free path, which is about 500 nanometers, and then calculate the dielectric strength from that, we get 10,000,000 V/mm , and the real electrical strength for liquids ranges from 20,000 to 40,000 V / mm.
The dielectric strength of liquids actually depends on the amount of gas in those liquids. Also, the dielectric strength depends on the condition of the electrode surfaces to which the voltage is applied. Breakdown into a liquid begins with the breakdown of small gas bubbles.
The gas has a much lower dielectric constant, so the voltage in the bubble turns out to be higher than in the surrounding liquid. In this case, the dielectric strength of the gas is lower. Bubble discharges lead to bubble growth and eventually liquid breakup occurs as a result of partial discharges in the bubbles.
Impurities play an important role in the breakdown development mechanism in liquid dielectrics. Consider, for example, transformer oil. Soot and water as conductive impurities reduce the dielectric strength transformer oil.
Although water usually does not mix with oil, its smallest droplets in the oil under the action of an electric field polarize, form circuits with increased electrical conductivity compared to the surrounding oil, and as a result, oil breakdown occurs along the circuit.
To determine the dielectric strength of liquids in laboratory conditions, hemispherical electrodes are used, the radius of which is several times greater than the distance between them. A uniform electric field is created in the gap between the electrodes. A typical distance is 2.5 mm.
For transformer oil, the breakdown voltage should not be less than 50,000 volts, and its best samples differ in the breakdown voltage value of 80,000 volts. At the same time, remember that in the impact ionization theory this voltage should have been 2,000,000 — 3,000,000 volts.
So, to increase the dielectric strength of a liquid dielectric, it is necessary:
-
Clean the liquid from solid conductive particles such as coal, soot, etc.;
-
Remove the water from the dielectric fluid;
-
Disinfect the liquid (evacuate);
-
Increase fluid pressure.
Dielectric strength of solid dielectrics
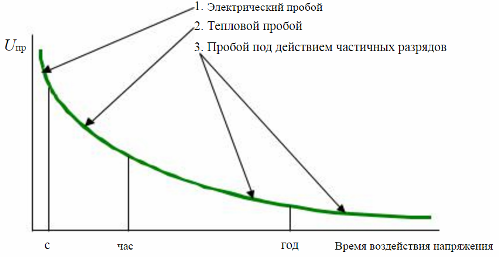
The dielectric strength of solid dielectrics is related to the time during which the breakdown voltage is applied. And depending on the time when the voltage is applied to the dielectric, and on the physical processes that occur at that time, they distinguish:
-
Electrical failure that occurs in fractions of seconds after voltage is applied;
-
Thermal collapse that occurs in seconds or even hours;
-
Breakdown due to partial discharges, exposure time may be more than a year.
The mechanism of the breakdown of a solid dielectric consists in the destruction of chemical bonds in a substance under the action of an applied voltage, with the transformation of the substance into a plasma. That is, we can talk about the proportionality between the electrical strength of a solid dielectric and the energy of its chemical bonds.
Solid dielectrics often exceed the dielectric strength of liquids and gases, for example, insulating glass has an electrical strength of about 70,000 V/mm, polyvinyl chloride — 40,000 V/mm, and polyethylene — 30,000 V/mm.
The cause of thermal breakdown lies in the heating of the dielectric due to dielectric losswhen the power loss energy exceeds the energy removed by the dielectric.
As the temperature increases, the number of carriers increases, the conductivity increases, the loss angle increases, and therefore the temperature increases even more and the dielectric strength decreases. As a result, due to the heating of the dielectric, the resulting failure occurs at a lower voltage than without heating, that is, if the failure was purely electrical.
