Corrosion resistance of metals
What is corrosion resistance?
The ability of a metal to resist corrosion is called corrosion resistance. This ability is determined by the rate of corrosion under certain conditions. Quantitative and qualitative characteristics are used to assess the degree of corrosion.

The qualitative characteristics are:
-
changing the appearance of the metal surface;
-
change in the microstructure of the metal.
The quantitative characteristics are:
-
time before the appearance of the first focus of corrosion;
-
the number of corrosion foci formed over a certain period of time;
-
metal thinning per unit time;
-
change in mass of metal per unit area per unit time;
-
the volume of gas absorbed or released during corrosion per unit surface per unit time;
-
electric current density for a given corrosion rate;
-
change in property over a period of time (mechanical properties, reflectivity, electrical resistance).
Different metals have different resistance to corrosion.To increase corrosion resistance, special methods are used: alloying for steel, chrome plating, aluminization, nickel plating, painting, zinc coating, passivation, etc.
Iron and steel

In the presence of oxygen and pure water, iron quickly corrodes, the reaction proceeds according to the formula:
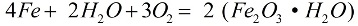
In the process of corrosion, a loose layer of rust covers the metal, and this layer does not at all protect it from further destruction, corrosion continues until the metal is completely destroyed. The more active corrosion of iron is caused by salt solutions: if even a little ammonium chloride (NH4Cl) is present in the air, the corrosion process will go much faster. In a weak solution of hydrochloric acid (HCl), the reaction will also proceed actively.
Nitric acid (HNO3) in a concentration above 50% will lead to passivation of the metal — it will be covered with a protective layer, albeit fragile. Vaporized nitric acid is safe for iron.
Sulfuric acid (H2SO4) in a concentration above 70% passivates iron, and if steel class St3 is stored in 90% sulfuric acid at a temperature of 40 ° C, then under these conditions the corrosion rate will not exceed 140 microns per year. If the temperature is 90 ° C, then the corrosion will continue at a 10 times higher rate. Sulfuric acid with an iron concentration of 50% will dissolve.
Phosphoric acid (H3PO4) will not corrode iron, nor will anhydrous organic solvents such as alkaline solutions, aqueous ammonia, dry Br2 and Cl2.
If you add one thousandth of sodium chromate to water, it will become an excellent iron corrosion inhibitor, like sodium hexametaphosphate. But chlorine ions (Cl-) remove the protective film from the iron and increase corrosion.The iron is technically pure, contains about 0.16% impurities and is highly resistant to corrosion.
Medium-alloyed and low-alloyed steels
Alloying additions of chromium, nickel or copper in low-alloyed and medium-alloyed steels increase their resistance to water and atmospheric corrosion. The more chromium, the higher the oxidation resistance of the steel. But if the chromium is less than 12%, then chemically active media will have a destructive effect on such steel.
High alloy steels
In high-alloyed steels, the alloying components are more than 10%. If the steel contains from 12 to 18% chromium, then such steel will withstand contact with almost any of the organic acids, with food, will be resistant to nitric acid (HNO3), bases, many salt solutions. In 25% formic acid (CH2O2) high alloy steel will corrode at a rate of about 2 mm per year. However, strong reducing agents, hydrochloric acid, chlorides and halogens will destroy high alloy steel.
Stainless steels that contain 8 to 11% nickel and 17 to 19% chromium are more resistant to corrosion than high chromium steels alone. Such steels withstand acidic oxidizing media, such as chromic acid or nitric acid, as well as strong alkaline.
Nickel as an additive will increase the steel's resistance to non-oxidizing environments, to atmospheric factors. But the environment is acidic, reducing and acidic with halogen ions, - they will destroy the passivating oxide layer, as a result, the steel will lose its resistance to acids.
Stainless steels with the addition of molybdenum in an amount of 1 to 4% have higher corrosion resistance than chrome-nickel steels.Molybdenum will give resistance to sulfuric and sulfuric acid, organic acids, sea water and halides.
Ferrosilicon (iron with an addition of 13 to 17% silicon), the so-called iron-silicon casting, has corrosion resistance due to the presence of an oxide film of SiO2 and which neither sulfuric, nor nitric, nor chromic acids can destroy, they only strengthen this protective film. But hydrochloric acid (HCl) will easily corrode ferrosilicon.
Nickel alloys and pure nickel
Nickel is resistant to many factors, both atmospheric and laboratory, to clean and salt water, to alkaline and neutral salts such as carbonates, acetates, chlorides, nitrates and sulfates. Non-oxygenated and non-hot organic acids will not harm nickel, as well as boiling concentrated alkaline potassium hydroxide (KOH) in a concentration of up to 60%.
Corrosion is caused by reducing and oxidizing media, oxidizing alkaline or acidic salts, oxidizing acids such as nitrogen, moist gaseous halogens, nitrogen oxides, and sulfur dioxide.
Monel metal (up to 67% nickel and up to 38% copper) is more acid resistant than pure nickel, but will not withstand the action of strong oxidizing acids. It differs in fairly high resistance to organic acids, to a significant amount of salt solutions. Atmospheric and water corrosion do not threaten monel metal; fluoride is also safe for him. Monel metal will safely withstand 40% boiling hydrogen fluoride (HF) like platinum.
Aluminum alloys and pure aluminum

Aluminum's protective oxide film makes it resistant to common oxidizers, acetic acid, fluorine, the atmosphere alone, and a significant amount of organic liquids.Technically pure aluminum, in which impurities are less than 0.5%, is very resistant to the action of hydrogen peroxide (H2O2).
It is destroyed by the action of caustic bases in a strongly reducing environment. Dilute sulfuric acid and oleum aren't terrible for aluminum, but medium-strength sulfuric acid will destroy it, as will hot nitric acid.
Hydrochloric acid can destroy aluminum's protective oxide film. Contact of aluminum with mercury or mercury salts is destructive for the former.
Pure aluminum is more resistant to corrosion than, for example, duralumin alloy (in which up to 5.5% copper, 0.5% magnesium and up to 1% manganese), which is less resistant to corrosion. Silumin (adding 11 to 14% silicon) is more stable in this regard.
Copper alloys and pure copper

Pure copper and its alloys do not corrode in salt water or air. Copper is not afraid of corrosion: dilute bases, dry NH3, neutral salts, dry gases and most organic solvents.
Alloys such as bronze, which contain a lot of copper, withstand exposure to acids, even cold concentrated or hot dilute sulfuric acid, or concentrated or dilute hydrochloric acid at room temperature (25 ° C).
In the absence of oxygen, copper does not corrode in contact with organic acids. Neither fluorine nor dry hydrogen fluoride has a destructive effect on copper.
But copper alloys and pure copper are corroded by various acids if oxygen is present, as well as in contact with wet NH3, some acid salts, wet gases such as acetylene, CO2, Cl2, SO2. Copper interacts easily with mercury. Brass (zinc and copper) is not highly resistant to corrosion.
Check more details here — Copper and aluminum in electrical engineering
Pure zinc

Clean water, like clean air, does not corrode zinc. But if there are salts, carbon dioxide or ammonia in water or air, then corrosion of zinc will begin. Zinc dissolves in bases, especially quickly — in nitric acid (HNO3), more slowly — in hydrochloric and sulfuric acids.
Organic solvents and petroleum products generally have no corrosive effect on zinc, but if contact is prolonged, for example with cracked gasoline, the acidity of the gasoline will increase as it oxidizes in the air and corrosion of the zinc will begin.
Pure lead

The high resistance of lead to water and atmospheric corrosion is a well-known fact. It does not corrode I lead and when in the soil. But if the water contains a lot of carbon dioxide, then the lead will dissolve in it, as lead bicarbonate is formed, which will already be soluble.
In general, lead is very resistant to neutral solutions, moderately resistant to alkaline solutions, as well as to some acids: sulfuric, phosphoric, chromic and sulfuric. With concentrated sulfuric acid (from 98%) at a temperature of 25 ° C, lead can slowly dissolve.
Hydrogen fluoride at a concentration of 48% will dissolve lead when heated. Lead reacts strongly with hydrochloric and nitric acids, with formic and acetic acid. Sulfuric acid will cover the lead with a slightly soluble layer of lead chloride (PbCl2) and further dissolution will not proceed. In concentrated nitric acid, the lead will also be coated with a layer of salt, but dilute nitric acid will dissolve the lead. Chlorides, carbonates and sulfates are not aggressive towards lead, while nitrate solutions are the opposite.
Pure titanium

Good corrosion resistance is a hallmark of titanium.It is not oxidized by strong oxidizers, withstands salt solutions, FeCl3, etc. Concentrated mineral acids will cause corrosion, but even boiling nitric acid in a concentration of less than 65%, sulfuric acid - up to 5%, hydrochloric acid - up to 5% - will not cause corrosion of titanium. Normal corrosion resistance to bases, alkaline salts and organic acids distinguishes titanium from other metals.
Pure zirconium

Zirconium is more resistant to sulfuric and hydrochloric acid than titanium, but less resistant to aquaregia and wet chlorine. It has high chemical resistance to most bases and acids, resistant to hydrogen peroxide (H2O2).
The action of certain chlorides, boiling concentrated hydrochloric acid, aqua regia (a mixture of concentrated nitric HNO3 (65-68 wt.%) and saline HCl (32-35 wt.%), hot concentrated sulfuric acid and fuming nitric acid-cause Regarding of corrosion, this is such a property of zirconium as hydrophobicity, that is, this metal is not wetted either by water or aqueous solutions.
Pure tantalum

Tantalum's excellent chemical resistance is similar to glass. Its dense oxide film protects the metal at temperatures up to 150 ° C from the action of chlorine, bromine, iodine. Most acids under normal conditions do not act on tantalum, even aquaregia and concentrated nitric acid do not cause corrosion. Alkaline solutions have practically no effect on tantalum, but hydrogen fluoride acts on it, and concentrated hot alkali solutions are used, alkaline melts are used to dissolve tantalum.
