How the process of converting solar energy into electrical energy works
Many of us have encountered solar cells in one way or another. Someone has used or is using solar panels to generate electricity for household purposes, someone uses a small solar panel to charge their favorite gadget in the field, and someone has certainly seen a small solar cell on a micro calculator. Some were even lucky enough to visit him solar power plant.
But have you ever wondered how the process of converting solar energy into electricity works? What physical phenomenon underlies the operation of all these solar cells? Let's turn to physics and understand the generation process in detail.
From the very beginning it is obvious that the source of energy here is sunlight or, scientifically speaking, Electrical energy is produced thanks to photons of solar radiation. These photons can be represented as a stream of elementary particles constantly moving from the Sun, each of which has energy, and therefore the entire light stream carries some kind of energy.
From every square meter of the Sun's surface, 63 MW of energy is continuously emitted in the form of radiation! The maximum intensity of this radiation falls on the range of the visible spectrum — wavelengths from 400 to 800 nm.
So, scientists have found that the energy density of the flow of sunlight at a distance from the Sun to the Earth is 149600000 kilometers, after passing through the atmosphere, and upon reaching the surface of our planet, an average of about 900 watts per square meter.
Here you can accept this energy and try to get electricity from it, that is, to convert the energy of the light flux of the sun into the energy of moving charged particles, in other words, in electricity.

To convert light into electricity, we need a photoelectric converter... Such converters are very common, they are found in free trade, these are the so-called solar cells - photovoltaic converters in the form of plates cut from silicon.
The best are monocrystalline, they have an efficiency of about 18%, that is, if the photon flow from the sun has an energy density of 900 W / m2, then you can count on receiving 160 W of electricity from a square meter of a battery assembled from such cells.
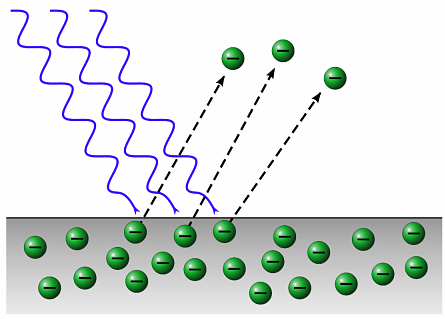
A phenomenon called the «photoelectric effect» works here. Photoelectric effect or photoelectric effect — This is the phenomenon of emission of electrons from a substance (the phenomenon of detachment of electrons from the atoms of a substance) under the influence of light or other electromagnetic radiation.
Already in 1900Max Planck, the father of quantum physics, suggested that light is emitted and absorbed by individual particles, or quanta, which later, in 1926, the chemist Gilbert Lewis would call "photons."
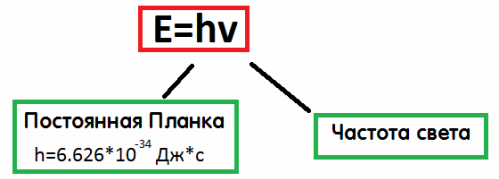
Each photon has an energy that can be determined by the formula E = hv — Planck's constant multiplied by the frequency of emission.
In accordance with Max Planck's idea, the phenomenon discovered in 1887 by Hertz and then thoroughly studied from 1888 to 1890 by Stoletov becomes explainable. Alexander Stoletov experimentally studied the photoelectric effect and established three laws of the photoelectric effect (Stoletov's laws):
-
At a constant spectral composition of electromagnetic radiation falling on the photocathode, the saturation photocurrent is proportional to the cathode irradiation (otherwise: the number of photoelectrons knocked out of the cathode in 1 s is directly proportional to the radiation intensity).
-
The maximum initial speed of the photoelectrons does not depend on the intensity of the incident light, but is determined only by its frequency.
-
For each substance there is a red limit of the photoelectric effect, that is, the minimum frequency of light (depending on the chemical nature of the substance and the state of the surface) below which the photoeffect is impossible.
Later, in 1905, Einstein would clarify the theory of the photoelectric effect. He will show how the quantum theory of light and the law of conservation and conversion of energy perfectly explain what happens and what is observed. Einstein would write the equation for the photoelectric effect, for which he won the Nobel Prize in 1921:
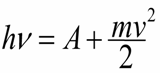
Work functions And here is the minimum work that an electron must do to leave an atom of a substance.The second term is the kinetic energy of the electron after exit.
That is, the photon is absorbed by the electron of the atom, therefore the kinetic energy of the electron in the atom increases by the amount of energy of the absorbed photon.
Part of this energy is spent on leaving the electron from the atom, the electron leaves the atom and gets the opportunity to move freely. And directed moving electrons are nothing more than electric current or photocurrent. As a result, we can talk about the appearance of EMF in a substance as a result of the photoelectric effect.
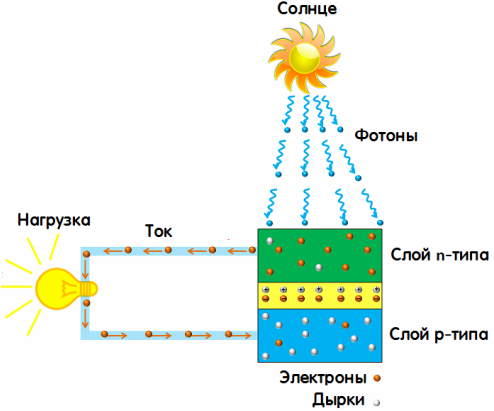
That is, the solar battery works thanks to the photoelectric effect operating in it. But where do the "knocked out" electrons go in the photovoltaic converter? Photovoltaic converter or solar cell or photocell is semiconductor, therefore, the photo effect occurs in it in an unusual way, it is an internal photo effect, and even has a special name "valve photo effect".
Under the influence of sunlight, a photoelectric effect occurs in the pn junction of a semiconductor and an EMF appears, but the electrons do not leave the photocell, everything happens in the blocking layer when the electrons leave one part of the body, passing to another part of it.
Silicon in the earth's crust is 30% of its mass, which is why it is used everywhere. The peculiarity of semiconductors in general lies in the fact that they are neither conductors nor dielectrics, their conductivity depends on the concentration of impurities, on temperature and on the effect of radiation.
The bandgap in a semiconductor is a few electron volts, and it's just the energy difference between the upper valence band level of the atoms, from which electrons are withdrawn, and the lower conduction level. Silicon has a bandgap of 1.12 eV—just what is needed to absorb solar radiation.
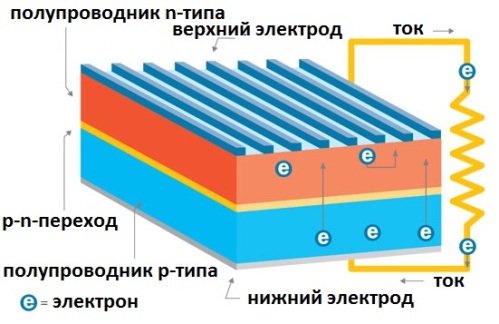
So pn junction. Doped silicon layers in the photocell form a pn junction. Here there is an energy barrier for electrons, they leave the valence band and move in only one direction, holes move in the opposite direction. This is how the current in the solar cell is obtained, that is, the generation of electricity from sunlight.
The pn junction, exposed to the action of photons, does not allow the charge carriers — electrons and holes — to move in a way other than just one direction, they separate and end up on opposite sides of the barrier. And when connected to the load circuit through the upper and lower electrodes, the photovoltaic converter, when exposed to sunlight, will create in the external circuit direct electric current.
