Coulomb's law and its application in electrical engineering
Just like in Newtonian mechanics, gravitational interaction is always carried out between tables with masses, similar to electrodynamics, electrical interaction is characteristic of bodies with electric charges. Electric charge is denoted by the symbol «q» or «Q».
We can even say that the concept of electric charge Q in electrodynamics is somewhat similar to the concept of gravitational mass M in mechanics. But unlike the gravitational mass, the electric charge characterizes the property of bodies and particles to enter into force electromagnetic interactions and these interactions, as you understand, are not gravitational.
Electric charges
Human experience in studying electrical phenomena contains many experimental results, and all these facts have allowed physicists to come to the following unambiguous conclusions about electrical charges:
1. Electric charges are of two types - conditionally they can be divided into positive and negative.
2.Electric charges can be transferred from one charged object to another: for example, by contacting bodies with each other - the charge between them can be separated. In this case, the electric charge is not a mandatory component of the body at all: under different conditions, the same object may have a charge of different magnitude and sign, or it may have no charge. Thus the charge is not something inherent in the carrier, and at the same time the charge cannot exist without the carrier.
3. While gravitating bodies always attract each other, electric charges can both attract each other and repel each other. Like charges mutually attract, like charges repel.
Charge carriers are electrons, protons and other elementary particles. There are two types of electric charges—positive and negative. The positive charges are those that appear on the glass rubbed with leather. Negative - Charges occurring on fur-rubbed amber. The authorities charged with the charges of the same name push back. Objects with opposite charges attract each other.
The law of conservation of electric charge is a fundamental law of nature, it reads like this: «the algebraic sum of charges of all bodies in an isolated system remains constant». This means that in a closed system, the appearance or disappearance of charges for only one sign is impossible.
The algebraic sum of charges in an isolated system is kept constant. Charge carriers can move from one body to another or move inside a body, in a molecule, atom. The charge is independent of the frame of reference.
Today, the scientific view is that originally charge carriers were elementary particles.Elementary particles neutrons (electrically neutral), protons (positively charged) and electrons (negatively charged) make up atoms.
The nuclei of atoms are made up of protons and neutrons, and electrons form the shells of atoms. The moduli of the charges of an electron and a proton are equal in magnitude to the elementary charge e, but in sign the charges of these particles are opposite to each other.
Interaction of Electric Charges — Coulomb's Law
As for the direct interaction of electric charges with each other, then in 1785 the French physicist Charles Coulomb experimentally established and described this basic law of electrostatics, the basic law of nature, which does not follow from any other laws. In his work, the scientist studies the interaction of stationary point-charged bodies and measures the forces of their mutual repulsion and attraction.
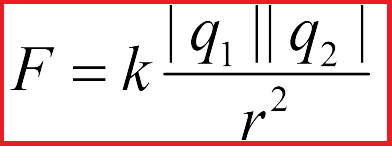
Coulomb experimentally established the following: «The forces of interaction of stationary charges are directly proportional to the product of the modules and inversely proportional to the square of the distance between them.»
This is the formulation of Coulomb's law. And although point charges do not exist in nature, only in terms of point charges can we talk about the distance between them, within this formulation of Coulomb's law.
In fact, if the distances between the bodies significantly exceed their sizes, then neither the size nor the shape of the charged bodies will particularly affect their interaction, which means that the bodies for this problem can fairly be considered point-like.
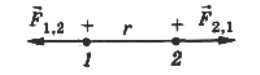
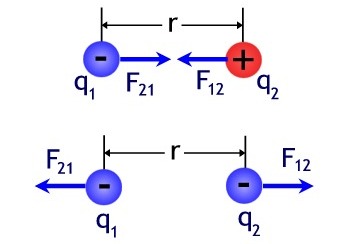
Let's look at an example. Let's hang some charged balls on strings.Because they are charged in some way, they will either repel or attract. Since the forces are directed along a straight line connecting these bodies, these are central forces.
To denote the forces acting on each of the charges from the other, we will write: F12 is the force of the second charge on the first, F21 is the force of the first charge on the second, r12 is the radius vector from the second point charge to the first. If the charges have the same sign, then the force F12 will be jointly directed to the radius vector, but if the charges have different signs, then the force F12 will be directed against the radius vector.
Using the law of interaction of point charges (Coulomb's Law), the interaction force can now be found for any point charges or point charge bodies. If the bodies are not point-shaped, they are mentally broken up into pastels of elements, each of which can be taken as a point charge.
After finding the forces acting between all the small elements, these forces add up geometrically—they find the resultant force. Elementary particles also interact with each other according to Coulomb's law, and to date no violations of this fundamental law of electrostatics have been observed.
Application of Coulomb's law in electrical engineering
There is no area in modern electrical engineering where Coulomb's law does not operate in one form or another. Starting with an electric current, ending with a simply charged capacitor. Especially those areas that deal with electrostatics — they are 100% related to Coulomb's law. Let's look at just a few examples.
The simplest case is the introduction of a dielectric.The force of interaction of charges in a vacuum is always greater than the force of interaction of the same charges under conditions when some kind of dielectric is placed between them.
The dielectric constant of a medium is precisely that value that allows you to quantitatively determine the values of the forces, regardless of the distance between the charges and their magnitudes. It is enough to divide the interaction force of charges in a vacuum by the dielectric constant of the introduced dielectric — we get the interaction force in the presence of a dielectric.

Sophisticated research equipment — a particle accelerator. The operation of charged particle accelerators is based on the phenomenon of interaction of an electric field and charged particles. The electric field does work in the accelerator, increasing the energy of the particle.
If we consider here the accelerated particle as a point charge, and the action of the accelerating electric field of the accelerator as the total force from other point charges, then in this case Coulomb's law is fully observed. The magnetic field directs the particle only through the Lorentz force, but does not change its energy, but only sets the trajectory for movement of particles in the accelerator.
Protective electrical structures. Important electrical installations are always equipped with something as simple at first glance as a lightning rod. And the lightning rod in its work also does not pass without observing Coulomb's law. During a thunderstorm, large induced charges appear on Earth — according to Coulomb's law, they are attracted in the direction of the thunderstorm cloud. The result is a strong electric field on the earth's surface.
The intensity of this field is particularly high near sharp conductors, and therefore a coronal discharge is ignited at the pointed end of the lightning rod — the charge from the Earth tends, obeying Coulomb's law, to be attracted by the opposite charge of the thunderbolt. cloud.
The air near the lightning rod is highly ionized as a result of the corona discharge. As a result, the strength of the electric field near the tip decreases (as well as inside any wire), induced charges cannot accumulate on the building, and the probability of lightning is reduced. If lightning happens to strike the lightning rod, then the charge will simply go to Earth and will not damage the installation.
