What is the difference between graphene and graphite?
A remarkable chemical element, carbon is one that conveniently sits at number 6 in the fourteenth group of the second period of the periodic table of chemical elements. Since ancient times, people have known diamond and graphite, two of the more than nine allotropic modifications of this element discovered so far. By the way, it is carbon that has the largest, compared to other substances, number of allotropic modifications known to modern science.
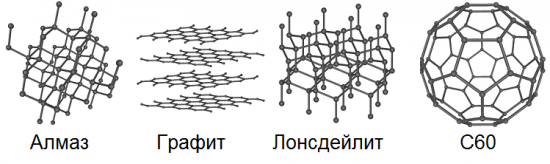
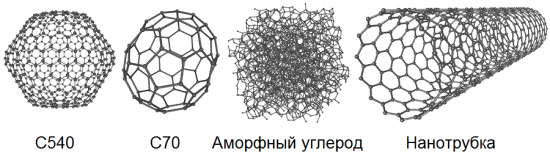
Allotropy implies the possibility of the existence in nature of the same chemical element in the form of two or more simple substances, the so-called allotropic forms or allotropic modifications, which cause differences in these substances both in structure and properties. So, carbon has 8 such basic forms: diamond, graphite, lonsdaleite, fullerenes (C60, C540 and C70), amorphous carbon and single-walled nanotube.
Among these forms of carbon there are completely different properties and character: soft and hard, transparent and opaque, cheap and expensive substances. However, let's compare two similar carbon modifications — graphite and graphene.

We are all familiar with graffiti since school.The lead of an ordinary pencil is exactly graphite. It is quite soft, slippery and greasy to the touch, the crystals are plates, the layers of atoms are located one above the other, therefore when rubbing, for example, on paper, individual flakes of the layered crystal structure of graphite easily peel off, leaving a characteristic dark trace on the paper.
Graphite conducts electric current well, its resistance is on average 11 Ohm * mm2 / m, but the conductivity of graphite is not the same due to the natural anisotropy of its crystals. Thus, the conductivity along the planes of the crystal is hundreds of times higher than the conductivity in these planes. The density of graphite is from 2.08 to 2.23 g / cm3.
In nature, graphite is formed at high temperatures in igneous and volcanic rocks, in skarns and pegmatites. It occurs in quartz veins with minerals in hydrothermal intermediate temperature polymetallic deposits. It is widely distributed in metamorphic rocks.
Thus, since 1907, the world's largest reserves of natural flake graphite have been developed on the island of Madagascar. The island consists of Precambrian metamorphic rocks that rise to the surface in a mountainous terrain with hypsometric marks of 4,000-4,600 feet. The graphite is found here in a belt 400 miles long and dominates the mountains in the eastern part of the center of the island.
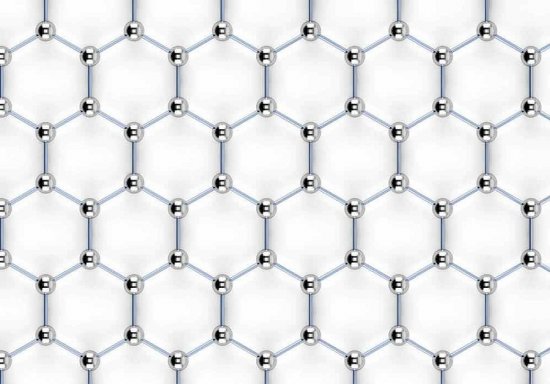
Graphene, unlike graphite, does not have a bulk crystal structure; it features a two-dimensional hexagonal crystal lattice, only one atom thick. In such an allotropic modification, carbon does not occur naturally at all, but can theoretically be obtained artificially. We can say that a plane deliberately separated from the multi-layered bulk crystal structure of graphite will be this very graphene.
Scientists were initially unable to obtain graphene in the form of a simple two-dimensional film, due to the instability of matter in this form. However, on a silicon oxide substrate (due to the bond with the dielectric layer) it was still possible to obtain one-atom-thick graphene: in 2004, Russian scientists Andrey Geim and Konstantin Novoselov of the University of Manchester published a report in Science on obtaining graphene in this way.
And even today, such simple methods of obtaining graphene for research, such as mechanical exfoliation of a carbon monolayer from a bulk graphite crystal using adhesive tape (and similar methods), are justified.
The researchers believe that thanks to their progress, a new class of graphene-based nanoelectronics will soon emerge, where field-effect transistors will be less than 10 nm thick. The fact is that the mobility of electrons in graphene is so high (10,000 cm2 / V * s) that it seems to be the most promising alternative to conventional silicon today.
High carrier mobility is the ability of electrons and holes to respond extremely quickly to the effect of applied electric fields, and this is extremely important for field-effect transistors, the basic operating unit of modern electronics.
There are also prospects for the creation of various biological and chemical sensors, as well as thin films for photovoltaic devices and touch screens. Despite all this, the thermal conductivity of graphene is 10 times higher than that of copper, and this criterion is always very important for electronics.