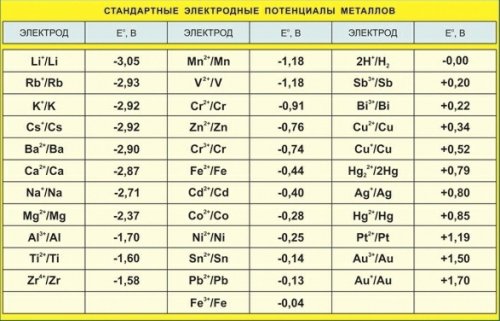
What is the potential of the electrode
Electrode potential or electrode potential of a metal is a potential difference that occurs at the metal-solution interface when a metal is immersed in an electrolyte solution as a result of the interaction of surface metal ion atoms located in the nodes of a crystal lattice with polar water molecules oriented to the surface of the electrode ... This is due to the formation of an electric double layer, that is, an asymmetric distribution of charged particles at the boundary.
The phenomenon of dissolution of metals in electrolytes is used in chemical sources of electricity. A metal plate smoked in a solution of its own salt, in one way or another, tends to dissolve in it. This tendency is sometimes called the dissolution elasticity of the metal.
A zinc plate immersed in a solution of zinc sulfate ZnTAKA4 gives zinc particles to the solution in the form of positively charged ions.Due to the fact that the pink atoms leave in the form of positively charged ions, an excess of free electrons is formed on the zinc plate and it becomes negatively charged, and an excess of positive ions is formed in the layer of liquid near the surface of the zinc, and therefore this layer is positively loaded. In this way, an electric double layer of spatially separated charges of opposite sign arises at the interface between the liquid and the metal.
These charges will oppose further passage of the metal into solution—the negative plates hold the positive metal ion, and the positive charge of the electrolyte pushes the metal ion back toward the plate. In other words, the electric field of the double layer at the metal-liquid interface counteracts the further transition of metal ions into solution. A balance is established between the forces of the tendency of the metal to go into solution, chemical in nature, and the electric forces that are opposed.
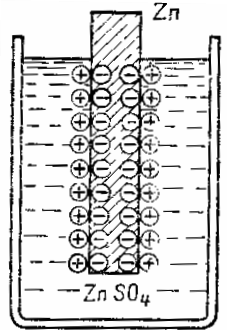
Diagram of the formation of an electric double layer at the interface between a metal and an electrolyte
Thus, due to dissolution in the electrolyte, the metal electrode acquires a certain electrode (in other words, electrochemical) potential with respect to the electrolyte, which depends on the material of the electrode and the composition of the electrolyte.
However, electrode potentials can be positive. This happens in cases where the positive ions of the solution pass to the electrode, charging it positively, and the electrolyte layer - negatively, for example, when a copper plate is immersed in a sufficiently concentrated solution of copper sulfate (CuSO)4).
The electric double layer can be likened to a capacitor, one of the plates of which is the metal surface and the other is a layer of ions in solution on the metal surface. Between the oppositely charged plates and there is a difference, or jump, in potential.
The potential jump at the electrode-solution interface can serve as a measure of the redox capacity of the system. However, it is impossible to measure such a potential jump or, equivalently, the potential difference between the two phases. But you can measure e. etc. c. elements composed of the electrodes we are interested in and some one (the same in all cases) electrode, the potential of which is conditionally assumed to be zero.
It has been measured, etc. s. will characterize the redox capacity of the electrode of interest relative to some conditional zero. The value thus obtained is called the internal potential of the metal.
To measure the electrode potential of any metal, it is necessary to place a second electrode in the electrolyte, which in turn will have a certain electrode potential, depending on its material. Therefore, only the algebraic sum of two electrode potentials can be measured directly.
For this reason, the electrode potentials of various materials are determined with respect to a standard (a hydrogen electrode, whose potential is usually taken to be zero.
Other reference electrodes whose potential relative to the hydrogen standard electrode is known can also be used for measurement. This potential is also found based on the measurement of e. etc. c. a circuit composed of a selected reference electrode and a standard hydrogen electrode.
If the studied electrode connected to a standard hydrogen electrode is negative, then the sign » -» is assigned to the internal potential, otherwise, the sign «+».
For example, the electrode potential of zinc -0.76 V, copper +0.34 V, silver +0.8 V, measured in this way in a solution of the corresponding metal salt, is determined by subtracting the more negative potential from the potential at -positive.
If two metal plates with different electrode potential are placed in the corresponding electrolyte, for example, in a solution of sulfuric acid (H2SO4) placed zinc (Zn) and copper (Cth), then a voltmeter connected to these plates will show a voltage between them slightly more than 1 V.
This voltage, in this case called e. etc. c. galvanic couple, will be due to the difference in electrode potentials of copper, which has a small positive potential, and zinc, which has a significant negative potential. Such a device is the simplest galvanic cell — the Volta cell.
In a galvanic cell, chemical energy is converted into electrical energy and with its help it is possible to perform electrical work due to the energy of a chemical reaction.
Measurement of e. etc. c. galvanic cells must be produced in the absence of current in the cell circuit. Otherwise, the measured e. etc. s. will be less than the value defined as the difference between the equilibrium potentials of the two electrodes… In fact, a certain concentration of electrons on the electrodes corresponds to the equilibrium potential: the more positive it is lower, the more negative it is higher. Accordingly, the structure of that part of the double layer that is in solution is also different.
Measurement of e. etc. witha cell without current flow is usually produced by the compensation method. To apply it, you need to have some standard e. etc. with The so-called normal element serves as such a standard. Most commonly they use Weston's mercury-cadmium normal cell, e.g. etc. with. which is equal to 1.01830 V at 20 ° C.