What is an electrolyte
Substances in which the electric current is due to the movement of ions, i.e. ionic conductivityare called electrolytes. Electrolytes belong to conductors of the second type, since the current in them is related to chemical processes, and not simply to the movement of electrons, as in metals.
Molecules of these substances in solution are capable of electrolytic dissociation, that is, they decompose when dissolved into positively charged (cations) and negatively charged (anions) ions. Solid electrolytes, ionic melts, and electrolyte solutions can be found in nature. Depending on the type of solvent, electrolytes are aqueous and non-aqueous, as well as a special type - polyelectrolytes.
Depending on the type of ions into which the substance decomposes when dissolved in water, electrolytes without H + and OH- ions (salt electrolytes), electrolytes with an abundance of H + ions (acids) and electrolytes with a predominance of OH- ions (base) can be isolated.
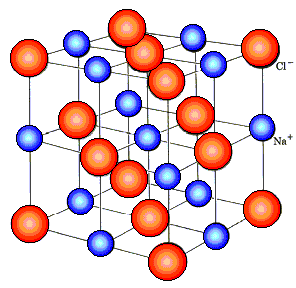
If an equal number of positive and negative ions are formed during the dissociation of electrolyte molecules, such an electrolyte is called symmetrical.Or asymmetric if the number of positive and negative ions in the solution is not the same. Examples of symmetrical electrolytes - KCl - 1,1-valent electrolyte and CaSO4 - 2,2-valent electrolyte. A representative of an asymmetric electrolyte is, for example, H2TAKA4 — a 1,2-valent electrolyte.

All electrolytes can be roughly divided into strong and weak, depending on their ability to dissociate. Strong electrolytes in dilute solutions decompose almost completely into ions. These include a large number of inorganic salts, some acids and bases in aqueous solutions or solvents with high dissociation power, such as alcohols, ketones or amides.
Weak electrolytes are only partially decomposed and are in dynamic equilibrium with undissociated molecules. These include a large number of organic acids as well as many bases in solvents.
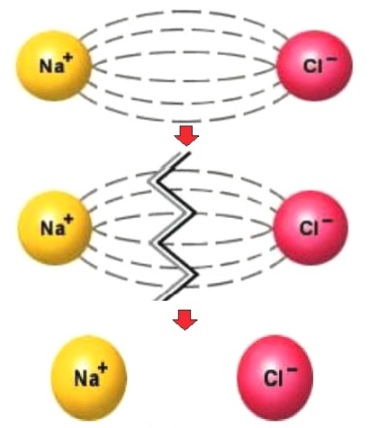
The degree of dissociation depends on several factors: temperature, concentration and type of solvent. So, the same electrolyte at different temperatures, or at the same temperature but in different solvents, will be dissociated to different degrees.
Since electrolytic dissociation, by definition, generates a larger number of particles in solution, it leads to significant differences in the physical properties of solutions of electrolytes and substances of different types: the osmotic pressure increases, the freezing temperature changes in relation to the solvent purity and others.
Electrolyte ions often participate in electrochemical processes and chemical reactions as independent kinetic units, independent of other ions present in the solution: on the electrodes immersed in the electrolyte, when the current passes through the electrolyte, oxidation-reduction reactions take place, the products of which are added to the electrolyte composition .
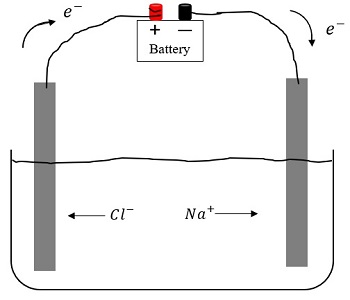
Thus, electrolytes are complex systems of substances that include ions, solvent molecules, undissociated solute molecules, ion pairs, and larger compounds. Therefore, the properties of electrolytes are determined by a number of factors: the nature of ion-molecular and ion-ion interactions, changes in the structure of the solvent in the presence of dissolved particles, etc.
Ions and molecules of polar electrolytes interact very actively with each other, which leads to the formation of solvation structures, the role of which becomes more significant with a decrease in the size of ions and an increase in their valences. The solvation energy is a measure of the interaction of electrolyte ions with solvent molecules.
Electrolytes, depending on their concentration, are: dilute solutions, transient and concentrated. Dilute solutions are similar in structure to a pure solvent, but the ions present disrupt this structure by their influence. Such weak solutions of strong electrolytes differ from ideal solutions in properties due to the electrostatic interaction between ions.
The transition region of concentration is characterized by a significant change in the structure of the solvent due to the influence of ions.At even higher concentration, most solvent molecules participate in solvation structures with ions, thus creating a solvent deficit.
The concentrated solution has a structure close to an ionic melt or crystalline solvate, characterized by high order and uniformity of ionic structures. These ionic structures bond with each other and with water molecules through complex interactions.
High-temperature and low-temperature regions of their properties, as well as high- and normal-pressure regions, are characteristic of electrolytes. As the pressure or temperature increases, the molar ordering of the solvent decreases and the influence of associative and solation effects on the properties of the solution weakens. And when the temperature drops below the melting point, some electrolytes go into a glassy state. An example of such an electrolyte is an aqueous solution of LiCl.
Today, electrolytes play a particularly important role in the world of technology and biology. In biological processes, electrolytes act as a medium for inorganic and organic synthesis, and in technology as a basis for electrochemical production.
Electrolysis, electrocatalysis, corrosion of metals, electrocrystallization — these phenomena occupy important places in many modern industries, especially in terms of energy and environmental protection.
See also: Production of hydrogen by electrolysis of water — technology and equipment



