Methods and instruments for measuring temperature
What is temperature
Temperature measurement is the subject of a theoretical and experimental discipline — thermometry, a part of which, covering temperatures above 500 ° C, is called pyrometry.
The most general strict definition of the concept of temperature, following the second law of thermodynamics, is formulated with the expression:
T = dQ /dC,
where T is the absolute temperature of an isolated thermodynamic system, dQ is the increment of heat transferred to that system, and dS is the increase in entropy of that system.
The above expression is interpreted as follows: temperature is a measure of the increase in heat transferred to an isolated thermodynamic system and corresponding to the increase in the entropy of the system that occurs in this case, or, in other words, to the increase in the disturbance of its state .
In statistical mechanics, which describes the phases of the system, taking into account the microprocesses occurring in the macrosystems, the concept of temperature is defined by expressing the distribution of the particles of a molecular system between a number of unoccupied energy levels (Gibbs distribution).
This definition (in accordance with the previous one) emphasizes the probabilistic, statistical aspect of the concept of temperature as the main parameter of the microphysical form of energy transfer from one body (or system) to another, i.e. chaotic thermal motion.
The lack of clarity of strict definitions of the concept of temperature, which are also valid only for thermodynamically balanced systems, has led to the widespread use of a "utilitarian" definition based on the essence of the phenomenon of energy transfer: temperature is the thermal state of a body or system characterized by its ability to exchange heat with another body (or system).
This formulation is applicable both to thermodynamically non-equilibrium systems and (with reservations) to the psychophysiological concept of «sensory» temperature, perceived directly by a person using the organs of thermal touch.
"Sensory" temperature is subjectively assessed by a person directly, but only qualitatively and in a relatively narrow interval, while physical temperature is measured quantitatively and objectively, with the help of measuring devices, but only indirectly - through the value of some physical quantity depending on the measured temperature .
Therefore, in the second case, some reference (reference) state of the temperature-dependent physical quantity selected for this purpose is established and a certain numerical temperature value is assigned to it, so that any change in the state of the selected physical quantity relative to the reference can be expressed in temperature units.
The set of temperature values corresponding to a series of successive changes in state (ie, a sequence of values) of a selected temperature-dependent quantity forms a temperature scale. The most common temperature scales are Celsius, Fahrenheit, Reaumur, Kelvin, and Rankine.
Kelvin and Celsius temperature scales
V 1730 French naturalist René Antoine Reumour (1683-1757), based on Amoton's suggestion, marked the melting point of ice on the thermometer as 0, and the boiling point of water as 80O. V 1742 NSVedic astronomer and physicist Anders Celsius (1701 — 1744), after two years of testing the Reaumur thermometer, discovered an error in the graduation of the scale.
It turned out that this depends largely on the atmospheric pressure. Celsius proposed to determine the pressure when calibrating the scale, and I divided the entire temperature range by 100, but assigned the mark 100 to the melting point of ice. Later, the Swedish Linnaeus or the German Stremmer (according to various sources) changed the designations of the control points.
Thus appeared the now widely used Celsius temperature scale. Its calibration is performed at normal atmospheric pressure of 1013.25 hPa.
Temperature scales were created by Fahrenheit, Reaumur, Newton (the latter inadvertently chose the temperature of the human body as a starting point.Well, the great ones are wrong!) And many others. They have not stood the test of time.
The Celsius temperature scale was adopted at the 1st General Conference on Weights and Measures in 1889. Currently, the degree Celsius is the official unit of temperature measurement established by the International Committee of Weights and Measures, but with some clarifications in the definition.
According to the above arguments, it is easy to conclude that the Celsius temperature scale is not the result of the activity of one person. Celsius was only one of the last researchers and inventors involved in its development. Until 1946, the scale was simply called a degree scale. It was only then that the International Committee of Weights and Measures assigned the name "degree Celsius" to the degree of degrees Celsius.
A few words about the working body of thermometers. The first creators of devices naturally sought to expand their range of action. The only liquid metal under normal conditions is mercury.
There was no choice. The melting point is -38.97 ° C, the boiling point is + 357.25 ° C. Of the volatile substances, wine or ethyl alcohol turned out to be the most available. Melting point — 114.2 ° C, boiling point + 78.46 ° C.
The created thermometers are suitable for measuring temperatures from -100 to + 300 ° C, which is enough to solve most practical problems. For example, the minimum air temperature is -89.2 ° C (Vostok station in Antarctica), and the maximum is + 59 ° C (Sahara desert). Most of the heat treatment processes of aqueous solutions took place at temperatures not higher than 100 °C.
The basic unit of measurement of thermodynamic temperature and at the same time one of the basic units International System of Units (SI) is the Kelvin degree.
The size (temperature gap) of 1 degree Kelvin is determined by the fact that the value of the thermodynamic temperature of the triple point of water is set exactly at 273.16 ° K.
This temperature, at which water exists in an equilibrium state in three phases: solid, liquid and gaseous, is taken as the main starting point because of its high reproducibility, an order of magnitude better than the reproducibility of the freezing and boiling points of water.
Measuring the triple point temperature of water is a technically difficult task. Therefore, as a standard, it was approved only in 1954 at the X General Conference on Weights and Measures.
The degree Celsius, in units of which the thermodynamic temperature can also be expressed, is exactly equal to the Kelvin in terms of temperature range, but the numerical value of any temperature in Celsius is 273.15 degrees higher than the value of the same temperature in Kelvin.
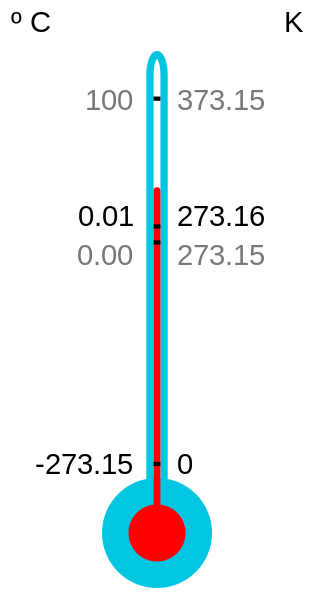
The size of 1 degree Kelvin (or 1 degree Celsius), determined by the numerical value of the temperature of the triple point of water, with modern measurement accuracy does not differ from its size determined (which was previously accepted) as a hundredth of the temperature difference between the freezing and boiling points of water.
Classification of methods and devices for measuring temperature
Measuring body or ambient temperature can be done in two fundamentally different indirect ways.
The first way leads to the measurement of the values of one of the temperature-dependent properties or state parameters of the body itself or the environment, the second - to the measurement of the values of the temperature-dependent properties or state parameters of the auxiliary body brought (directly or indirectly) to a state of thermal equilibrium with the body or environment whose temperature is measured...
An auxiliary body is called that serves these purposes and is a sensor of a complete temperature measuring device thermometric (pyrometric) probe or thermal detector… Therefore, all methods and devices for measuring temperature are divided into two fundamentally different groups: without probing and probe.
The thermal detector or any additional device of the device may be brought into direct mechanical contact with the body or medium whose temperature is measured, or only "optical" contact may be made between them.
Depending on this, all methods and tools for measuring temperature are divided into contact and non-contact. Probe contact and contactless methods and devices are of greatest practical importance.
Temperature measurement errors
All contact, mostly drilling, methods of temperature measurement, unlike other methods, are characterized by the so-called thermal or thermal methodological errors due to the fact that a complete probe thermometer (or pyrometer) measures the temperature value of only the sensitive part of the thermal detector, averaged over the surface or volume of that part.
Meanwhile, this temperature, as a rule, does not coincide with the measured one, since the thermal detector inevitably distorts the temperature field in which it is introduced. When measuring a stationary constant temperature of a body or environment, a certain mode of heat exchange is established between it and the thermal receiver.
The constant temperature difference between the thermal detector and the measured temperature of the body or environment characterizes the static thermal error in temperature measurement.
If the measured temperature changes, then the thermal error is a function of time. Such a dynamic error can be considered as consisting of a constant part, equivalent to the static error, and a variable part.
The latter arises because with each change in heat transfer between a body or medium whose temperature is measured, a new mode of heat transfer is not immediately established. The residual distortion of thermometer or pyrometer readings, which is a function of time, is characterized by the thermal inertia of the thermometer.
Thermal errors and thermal inertia of a thermal detector depend on the same factors as heat exchange between a body or environment and a thermal detector: on the temperatures of the thermal detector and the body or environment, on their size, composition (and hence properties ) and condition, by design, dimensions, geometric shape, state of the surface and properties of the materials of the thermal detector and the bodies around it, from their arrangement, according to which law the measured temperature of the body or environment changes over time.
Thermal methodological errors in temperature measurement, as a rule, are several times higher than the instrumental errors of thermometers and pyrometers. Their reduction is achieved by using rational methods of temperature measurement and constructions of thermal detectors and by appropriate installation of the latter at the places of use.
The improvement of heat transfer between the thermal receiver and the environment or the body whose temperature is measured is achieved by forcing beneficial and suppressing harmful factors of heat transfer.
For example, when measuring the temperature of a gas in a closed volume, the convective heat exchange of the thermal detector with the gas is increased, creating a rapid flow of gas around the thermal detector (a "suction" thermocouple), and radiant heat exchange with the walls of the volume is reduced, shielding the thermal detector ("shielded" thermocouple).
To reduce thermal inertia in thermometers and pyrometers with an electrical output signal, special circuits are also used that artificially reduce the signal rise time with a rapid change in the measured temperature.
Non-contact methods of temperature measurement
The possibility of using contact methods in measurements is determined not only by the distortion of the measured temperature by the contact thermal detector, but also by the real physico-chemical characteristics of the materials of the thermal detector (corrosion and mechanical resistance, heat resistance, etc.).
Non-contact measurement methods are free from these limitations. However, the most important of them, i.e.based on the laws of temperature radiation, special errors are inherent due to the fact that the laws used are exactly valid only for an absolutely black emitter, from which all real physical emitters (bodies and carriers) differ more or less in terms of radiation Properties.
According to Kirchhoff's laws of radiation, any physical body emits less energy than a black body heated to the same temperature as the physical body.
Therefore, a temperature measuring device calibrated against a black emitter, when measuring the temperature of a real physical emitter, will show a temperature lower than the actual one, namely the temperature at which the property of the black emitter used in calibration (radiative energy, its brightness, its spectral composition, etc.), matches in value with the property of a physical radiator at a given actual temperature to be determined. The measured underestimated pseudo temperature is called the black temperature.
Different measurement methods lead to different, as a rule, non-matching black temperatures: a radiation pyrometer shows integral or radiation, an optical pyrometer - brightness, a color pyrometer - color black temperatures.
The transition from measured blacks to actual temperatures is done graphically or analytically if the emissivity of the object whose temperature is measured is known.
The emissivity is the ratio of the values of the physical and black emitters used to measure the radiative properties that have the same temperature: with the radiative method, the emissivity is equal to the ratio of the total (across the spectrum) energies, with the optical method, the spectral emissivity ability is equal to the ratio of the spectral densities of the glow. All other things being equal, the smallest emitter non-blackness errors are given by a color pyrometer.
A radical solution to the problem of measuring the actual temperature of a non-black emitter by radiant methods is achieved by the arts by creating conditions for it to turn it into a black emitter (for example, by placing it in a practically closed cavity).
In some special cases, it is possible to measure the actual temperature of a non-black emitter with conventional radiation pyrometers using special temperature measurement techniques (for example, illumination, in three-wavelength beams, in polarized light, etc.).
General instruments for measuring temperature
The huge range of measured temperatures and an inexhaustible number of different conditions and objects of measurement determine an extraordinary variety and variety of methods and devices for measuring temperature.
The most common instruments for measuring temperature are:
- Thermoelectric pyrometers (thermometers);
- electric resistance thermometers;
- Radiation pyrometers;
- Optical absorption pyrometers;
- Optical brightness pyrometers;
- Color pyrometers;
- Liquid expansion thermometers;
- Gauge thermometers;
- Vapor thermometers;
- Gas condensation thermometers;
- Stick dilatometric thermometers;
- Bimetallic thermometers;
- Acoustic thermometers;
- Calorimetric pyrometers-pyroscopes;
- Thermal paints;
- Paramagnetic salt thermometers.
The most popular electrical devices for measuring temperature:
See also: Advantages and disadvantages of different temperature sensors
The many types of instruments listed above are used for measurements by various methods. For example, a thermoelectric thermometer is used:
- for contact measurement of the temperature of environments and bodies, as well as surfaces of the latter, without or in combination with devices that correct the thermal imbalance of the thermal detector and the measurement object;
- for non-contact temperature measurement by radiation and some spectroscopic methods;
- for mixed (contact-non-contact)-measurement of the temperature of the liquid metal by the gas cavity method (measurement of the radiation temperature of a gas bubble blown into the liquid metal at the end of a tube immersed in it with a radiation pyrometer).
At the same time, many temperature measurement methods can be applied with devices of various types.

For example, outdoor and indoor air temperature can be measured by devices of at least 15 types. The photo shows a bimetallic thermometer.
The world's largest thermometer in Baker, California
Application of temperature measuring instruments:
Measurement of surface temperatures with thermocouples
Non-contact temperature measurement during operation of electrical equipment