Dielectrics and their properties, polarization and breakdown strength of dielectrics
Substances (bodies) with negligible electrical conductivity are called dielectrics or insulators.
Dielectrics or non-conductors represent a large class of substances used in electrical engineering that are important for practical purposes. They serve to insulate electric circuits, as well as to give special properties to electrical devices, which enable more complete use of the volume and weight of the materials from which they are made.
Dielectrics can be substances in all aggregate states: gaseous, liquid and solid. In practice, air, carbon dioxide, hydrogen are used as gaseous dielectrics both in normal and compressed state.
All these gases have almost infinite resistance. The electrical properties of gases are isotropic. From liquid substances, chemically pure water, many organic substances, natural and artificial oils (transformer oil, owl, etc.).
Liquid dielectrics also have isotropic properties.The high insulating qualities of these substances depend on their purity.
For example, the insulating properties of transformer oil decrease when moisture is absorbed from the air. The most widely used in practice are solid dielectrics. They include substances of inorganic (porcelain, quartz, marble, mica, glass, etc.) and organic (paper, amber, rubber, various artificial organic substances) origin.
Most of these substances have high electrical and mechanical properties and are used for insulation of electrical appliancesintended for internal and external use.
A number of substances retain their high insulating properties not only at normal but also at elevated temperatures (silicon, quartz, silicon silicon compounds). Solid and liquid dielectrics have a certain amount of free electrons, which is why the resistance of a good dielectric is about 1015 - 1016 ohm x m.
Under certain conditions, the separation of molecules into ions occurs in dielectrics (for example, under the influence of high temperature or in a strong field), in this case dielectrics lose their insulating properties and become drivers.
Dielectrics have the property of being polarized and long-term existence is possible in them. electrostatic field.
A distinctive feature of all dielectrics is not only the high resistance to the passage of electric current, determined by the presence in them of a small number electrons, freely moving through the entire volume of the dielectric, but also a change in their properties under the action of an electric field, which is called polarization. Polarization has a large effect on the electric field in a dielectric.
One of the main examples of the use of dielectrics in electrical practice is the isolation of elements of electrical devices from the ground and from each other, due to which the destruction of the insulation disrupts the normal operation of electrical installations and leads to accidents.
To avoid this, in the design of electrical machines and installations, the insulation of individual elements is chosen so that, on the one hand, the field strength in the dielectrics does not exceed their dielectric strength anywhere, and on the other hand, this insulation in the individual connections of the devices is use as fully as possible (no excess stock).
To do this, you must first know how the electric field is distributed in the device. Then, by choosing the appropriate materials and their thickness, the above problem can be solved satisfactorily.

Dielectric polarization
If an electric field is created in a vacuum, then the magnitude and direction of the field strength vector at a given point depends only on the magnitude and location of the charges that create the field. If the field is created in any dielectric, then physical processes occur in the molecules of the latter that affect the electric field.
Under the action of electric field forces, electrons in orbits are displaced in the direction opposite to the field. As a result, previously neutral molecules become dipoles with equal charges on the nucleus and electrons in the orbits. This phenomenon is called dielectric polarization... When the field disappears, the displacement also disappears. The molecules become electrically neutral again.
Polarized molecules - dipoles create their own electric field, the direction of which is opposite to the direction of the main (external) field, therefore the additional field, combining with the main one, weakens it.
The more polarized the dielectric, the weaker the resulting field, the lower its intensity at any point for the same charges that create the main field, and therefore the dielectric constant of such a dielectric is greater.
If the dielectric is in an alternating electric field, the displacement of the electrons also becomes alternating. This process leads to an increase in the movement of particles and therefore to heating of the dielectric.
The more often the electric field changes, the more the dielectric heats up. In practice, this phenomenon is used to heat wet materials to dry them or to obtain chemical reactions that occur at elevated temperatures.
Read also: What is dielectric loss because of what happens
Polar and non-polar dielectrics
Although dielectrics practically do not conduct electricity, nevertheless, under the influence of an electric field, they change their properties. Depending on the structure of the molecules and the nature of the effect on them of the electric field, dielectrics are divided into two types: non-polar and polar (with electronic and orientational polarization).
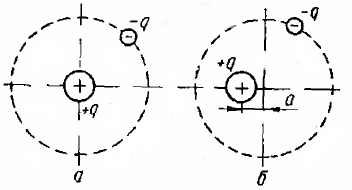
In non-polar dielectrics, if not in an electric field, the electrons spin in orbits with a center coinciding with the center of the nucleus. Therefore, the action of these electrons can be seen as the action of negative charges located in the center of the nucleus.Since the centers of action of positively charged particles — protons — are concentrated in the center of the nucleus, in outer space the atom is perceived as electrically neutral.
When these substances are introduced into the electrostatic field, the electrons are displaced under the influence of the field forces, and the centers of action of the electrons and protons do not coincide. In outer space, the atom in this case is perceived as a dipole, that is, as a system of two equal different point charges -q and + q, located from each other at a certain small distance a, equal to the displacement of the center of the electron orbit relative to the center of the nucleus.
In such a system, the positive charge turns out to be displaced in the direction of the field strength, the negative in the opposite direction. The greater the strength of the external field, the greater the relative displacement of the charges in each molecule.
When the field disappears, the electrons return to their original states of motion relative to the atomic nucleus and the dielectric becomes neutral again. The above change in the properties of a dielectric under the influence of a field is called electronic polarization.
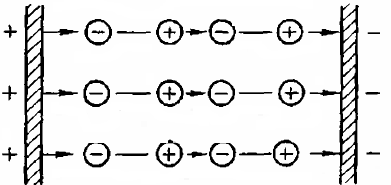
In polar dielectrics, the molecules are dipoles. Being in chaotic thermal motion, the dipole moment changes its position all the time. This leads to the compensation of the fields of the dipoles of individual molecules and to the fact that outside the dielectric, when there is no external field, there is no macroscopic field.
When these substances are exposed to an external electrostatic field, the dipoles will rotate and position their axes along the field. This fully ordered arrangement will be hindered by thermal motion.
At low field strength, only rotation of the dipoles occurs at a certain angle in the direction of the field, which is determined by the balance between the action of the electric field and the effect of thermal motion.
As the field strength increases, the rotation of the molecules and, accordingly, the degree of polarization increase. In such cases, the distance a between the dipole charges is determined by the average value of the projections of the dipole axes onto the direction of the field strength. In addition to this type of polarization, which is called orientational, there is also an electronic polarization in these dielectrics caused by the displacement of charges.

The polarization patterns described above are basic for all insulating substances: gaseous, liquid and solid. In liquid and solid dielectrics, where the average distances between molecules are smaller than in gases, the phenomenon of polarization is complicated, because in addition to the shift of the center of the electron orbit relative to the nucleus or the rotation of the polar dipoles, there is also an interaction between the molecules.
Since in the mass of a dielectric, individual atoms and molecules are only polarized, and do not break up into positively and negatively charged ions, in each element of the volume of a polarized dielectric, the charges of both signs are equal. Therefore, the dielectric throughout its volume remains electrically neutral.
Exceptions are the charges of the poles of the molecules located on the boundary surfaces of the dielectric. Such charges form thin charged layers at these surfaces. In a homogeneous medium, the phenomenon of polarization can be represented as a harmonic arrangement of dipoles.
The breakdown strength of dielectrics
Under normal conditions, the dielectric has negligible electrical conductivity… This property remains until the electric field strength is increased to a certain limiting value for each dielectric.
In a strong electric field, the molecules of the dielectric split into ions, and the body, which was a dielectric in a weak field, becomes a conductor.
The strength of the electric field at which the ionization of dielectric molecules begins is called the breakdown voltage (electrical strength) of the dielectric.
It is called the magnitude of the electric field strength that is allowed in a dielectric when it is used in electrical installations allowable voltage... The allowable voltage is usually several times less than the breaking voltage. The ratio of the breakdown voltage to the permissible safety margin is determined... The best non-conductors (dielectrics) are vacuum and gases, especially at high pressure.
Dielectric failure
Breakdown occurs differently in gaseous, liquid and solid substances and depends on a number of conditions: on the homogeneity of the dielectric, pressure, temperature, humidity, thickness of the dielectric, etc. Therefore, when determining the value of the dielectric strength, these conditions are usually provided .
For materials working, for example, in closed rooms and not exposed to atmospheric influences, normal conditions are established (for example, temperature + 20 ° C, pressure 760 mm). Humidity also normalizes, sometimes frequency, etc.
Gases have relatively low electrical strength. So the breakdown gradient of air under normal conditions is 30 kV / cm.The advantage of gases is that after their destruction, their insulating properties are quickly restored.
Liquid dielectrics have a slightly higher electrical strength. A distinctive feature of liquids is the good removal of heat from devices that are heated when the current passes through the wires. The presence of impurities, in particular water, significantly reduces the dielectric strength of liquid dielectrics. In liquids, as in gases, their insulating properties are restored after destruction.
Solid dielectrics represent a wide class of insulating materials, both natural and man-made. These dielectrics have a wide variety of electrical and mechanical properties.
The use of this or that material depends on the insulation requirements of the given installation and the conditions of its operation. Mica, glass, paraffin, ebonite, as well as various fibrous and synthetic organic substances, bakelite, getinax, etc. They are characterized by high electrical strength.
If, in addition to the requirement for a high breakdown gradient, a requirement for high mechanical strength is imposed on the material (for example, in support and suspension insulators, to protect equipment from mechanical stress), electrical porcelain is widely used.
The table shows the breakdown strength values (under normal conditions and at a constant constant zero) of some of the most common dielectrics.
Dielectric breakdown strength values
Material Breakdown voltage, kv / mm Paper impregnated with paraffin 10.0-25.0 Air 3.0 Mineral oil 6.0 -15.0 Marble 3.0 — 4.0 Mikanite 15.0 — 20.0 Electrical cardboard 9 .0 — 14.0 Mica 80.0 — 200.0 Glass 10.0 — 40.0 Porcelain 6.0 — 7.5 Slate 1.5 — 3.0






