Semiconductor materials — germanium and silicon
 Semiconductors represent a vast area of materials that differ from each other with a wide variety of electrical and physical properties, as well as with a wide variety of chemical composition, which determines different purposes in their technical use.
Semiconductors represent a vast area of materials that differ from each other with a wide variety of electrical and physical properties, as well as with a wide variety of chemical composition, which determines different purposes in their technical use.
By chemical nature, modern semiconductor materials can be classified into the following four main groups:
1. Crystalline semiconductor materials made up of atoms or molecules of a single element. Such materials are currently widely used germanium, silicon, selenium, boron, silicon carbide, etc.
2. Oxide crystalline semiconductor materials, i.e. metal oxide materials. The main ones are: copper oxide, zinc oxide, cadmium oxide, titanium dioxide, nickel oxide, etc. This group also includes materials based on barium titanate, strontium, zinc and other inorganic compounds with various small additives.
3. Crystalline semiconductor materials based on compounds of atoms from the third and fifth groups of Mendeleev's system of elements. Examples of such materials are indium, gallium and aluminum antimonides, i.e.compounds of antimony with indium, gallium, and aluminum. These were called intermetallic compounds.
4. Crystalline semiconductor materials based on compounds of sulfur, selenium and tellurium on the one hand and copper, cadmium and pig Ca on the other. Such compounds are called, respectively: sulfides, selenides and tellurides.
 All semiconductor materials, as already mentioned, can be divided by crystal structure into two groups. Some materials are made in the form of large single crystals (single crystals), from which plates of various sizes are cut in certain crystal directions for use in rectifiers, amplifiers, photocells.
All semiconductor materials, as already mentioned, can be divided by crystal structure into two groups. Some materials are made in the form of large single crystals (single crystals), from which plates of various sizes are cut in certain crystal directions for use in rectifiers, amplifiers, photocells.
Such materials make up the group of single crystal semiconductors... The most common single crystal materials are germanium and silicon. RMethods have been developed for the production of single crystals of silicon carbide, single crystals of intermetallic compounds.
Other semiconductor materials are a mixture of very small crystals randomly soldered together. Such materials are called polycrystalline... Representatives of polycrystalline semiconductor materials are selenium and silicon carbide, as well as materials made of various oxides using ceramic technology.
Consider widely used semiconductor materials.
Germanium — an element of the fourth group of Mendeleev's periodic system of elements. Germanium has a bright silver color. The melting point of germanium is 937.2 ° C. It is often found in nature, but in very small quantities. The presence of germanium is found in zinc ores and in the ash of various coals. The main source of germanium production is coal ash and waste from metallurgical plants.

Rice. 1. Germanium
Germanium ingot, obtained as a result of a number of chemical operations, is not yet a substance suitable for the manufacture of semiconductor devices from it. It contains insoluble impurities, is not yet a single crystal, and does not have an additive introduced into it that determines the required type of electrical conductivity.
It is widely used to clean the ingot from insoluble impurities zone melting method... This method can be used to remove only those impurities that dissolve differently in a given solid semiconductor and in its melt.
Germanium is very hard but extremely brittle and shatters into small pieces on impact. However, using a diamond saw or other devices, it can be cut into thin slices. Domestic industry produces alloyed germanium with electronic conductivity various grades with resistivity from 0.003 to 45 ohm NS cm and germanium alloyed with electrical conductivity of holes with resistivity from 0.4 to 5.5 ohm NS cm and above. The specific resistance of pure germanium at room temperature ρ = 60 ohm NS cm.
Germanium as a semiconductor material is widely used not only for diodes and triodes, it is used to make power rectifiers for high currents, various sensors used to measure magnetic field strength, resistance thermometers for low temperatures, etc.
Silicon widely distributed in nature. It, like germanium, is an element of the fourth group of the Mendeleev system of elements and has the same crystal (cubic) structure. Polished silicon takes on the metallic luster of steel.
Silicon does not occur naturally in the free state, although it is the second most abundant element on Earth, forming the basis of quartz and other minerals. Silicon can be isolated in its elemental form by high-temperature reduction of SiO2 carbon. At the same time, the purity of silicon after acid treatment is ~ 99.8%, and for semiconductor instrumental devices in this form, it is not used.
High-purity silicon is obtained from its previously well-purified volatile compounds (halides, silanes) either by their high-temperature reduction with zinc or hydrogen, or by their thermal decomposition. Released during the reaction, silicon is deposited on the walls of the reaction chamber or on a special heating element — most often on a rod made of high purity silicon.

Rice. 2. Silicon
Like germanium, silicon is brittle. Its melting point is significantly higher than that of germanium: 1423 ° C. The resistance of pure silicon at room temperature ρ = 3 NS 105 ohm-see
Since the melting point of silicon is much higher than that of germanium, the graphite crucible is replaced by a quartz crucible, because graphite at high temperatures can react with silicon to form silicon carbide. Additionally, graphite contaminants can enter molten silicon.
The industry produces semiconductor doped silicon with electronic conductivity (various grades) with resistivity from 0.01 to 35 ohm x cm and hole conductivity also of various grades with resistivity from 0.05 to 35 ohm x cm.
Silicon, like germanium, is widely used in the manufacture of many semiconductor devices.In the silicon rectifier, higher reverse voltages and operating temperatures (130 — 180 ° C) are achieved than in the germanium rectifiers (80 ° C). The point and plane are made of silicon diodes and triodes, photocells, and other semiconductor devices.
In fig. 3 shows the dependences of the resistance of germanium and silicon of both types on the concentration of impurities in them.
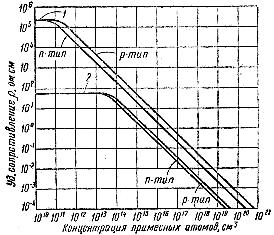
Rice. 3. Influence of the concentration of impurities on the resistance of germanium and silicon at room temperature: 1 — silicon, 2 — germanium
The curves in the figure show that impurities have a huge effect on the resistance: in germanium, it changes from the internal resistance value of 60 ohm x cm to 10-4 ohm x cm, that is, by 5 x 105 times, and for silicon by 3 x 103 to 10-4 ohm x cm, i.e. in 3 x 109 once.
As a material for the production of non-linear resistors, the polycrystalline material is particularly widely used - silicon carbide.
Rice. 4. Silicon carbide
Valve limiters for power lines are made of silicon carbide - devices that protect the power line from overvoltage. In them, disks made of a non-linear semiconductor (silicon carbide) pass current to the ground under the action of surge waves occurring in the line. As a result, normal operation of the line is restored. At operating voltage, the lines of resistance of these disks increase and the leakage current from the line to ground stops.
Silicon carbide is produced artificially — by heat treatment of a mixture of quartz sand with coal at high temperature (2000 ° C).
Depending on the additives introduced, two main types of silicon carbide are formed: green and black.They differ from each other in the type of electrical conductivity, namely: green silicon carbide throws n-type electrical conductivity, and black - with p-type conductivity.
For valve restrictors silicon carbide is used to produce discs with a diameter of 55 to 150 mm and a height of 20 to 60 mm. In a valve stop, silicon carbide discs are connected in series with each other and with spark gaps. The system consisting of discs and spark plugs is compressed by a coil spring. With a bolt, the arrester is connected to power line conductor, and ° C the other side of the arrester is connected by a wire to the ground. All parts of the fuse are placed in a porcelain case.
At normal transmission line voltage, the valve does not pass line current. At increased voltages (surges) created by atmospheric electricity or internal surges, spark gaps are created and the valve discs will be under high voltage.
Their resistance will drop sharply, which will ensure current leakage from the line to ground. The high current passed will reduce the voltage to normal and the resistance in the valve discs will increase. The valve will be closed, that is, the operating current of the line will not be transmitted to them.
Silicon carbide is also used in semiconductor rectifiers operating at high operating temperatures (up to 500 °C).
